Structures of human SGLT in the occluded state reveal conformational changes during sugar transport
- PMID: 37217492
- PMCID: PMC10203128
- DOI: 10.1038/s41467-023-38720-1
Structures of human SGLT in the occluded state reveal conformational changes during sugar transport
Abstract
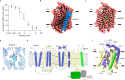
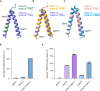
Sodium-Glucose Cotransporters (SGLT) mediate the uphill uptake of extracellular sugars and play fundamental roles in sugar metabolism. Although their structures in inward-open and outward-open conformations are emerging from structural studies, the trajectory of how SGLTs transit from the outward-facing to the inward-facing conformation remains unknown. Here, we present the cryo-EM structures of human SGLT1 and SGLT2 in the substrate-bound state. Both structures show an occluded conformation, with not only the extracellular gate but also the intracellular gate tightly sealed. The sugar substrate are caged inside a cavity surrounded by TM1, TM2, TM3, TM6, TM7, and TM10. Further structural analysis reveals the conformational changes associated with the binding and release of substrates. These structures fill a gap in our understanding of the structural mechanisms of SGLT transporters.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Mohebi, R. & Januzzi, J. L. Cardiovascular benefit of SGLT2 inhibitors. Crit. Rev. Clin. Lab. Sci. 59, 142–155 (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

