Flax fiber based semicarbazide biosorbent for removal of Cr(VI) and Alizarin Red S dye from wastewater
- PMID: 37217542
- PMCID: PMC10203277
- DOI: 10.1038/s41598-023-34523-y
Flax fiber based semicarbazide biosorbent for removal of Cr(VI) and Alizarin Red S dye from wastewater
Abstract
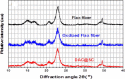
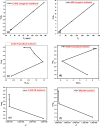
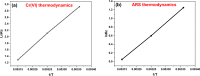
In the present study, flax fiber based semicarbazide biosorbent was prepared in two successive steps. In the first step, flax fibers were oxidized using potassium periodate (KIO4) to yield diadehyde cellulose (DAC). Dialdehyde cellulose was, then, refluxed with semicarbazide.HCl to produce the semicarbazide functionalized dialdehyde cellulose (DAC@SC). The prepared DAC@SC biosorbent was characterized using Brunauer, Emmett and Teller (BET) and N2 adsorption isotherm, point of zero charge (pHPZC), elemental analysis (C:H:N), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) analyses. The DAC@SC biosorbent was applied for the removal of the hexavalent chromium (Cr(VI)) ions and the alizarin red S (ARS) anionic dye (individually and in mixture). Experimental variables such as temperature, pH, and concentrations were optimized in detail. The monolayer adsorption capacities from the Langmuir isotherm model were 97.4 mg/g and 18.84 for Cr(VI) and ARS, respectively. The adsorption kinetics of DAC@SC indicated that the adsorption process fit PSO kinetic model. The obtained negative values of ΔG and ΔH indicated that the adsorption of Cr(VI) and ARS onto DAC@SC is a spontaneous and exothermic process. The DAC@SC biocomposite was successfully applied for the removal of Cr(VI) and ARS from synthetic effluents and real wastewater samples with a recovery (R, %) more than 90%. The prepared DAC@SC was regenerated using 0.1 M K2CO3 eluent. The plausible adsorption mechanism of Cr(VI) and ARS onto the surface of DAC@SC biocomposite was elucidated.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


















References
-
- Aragaw TA, Bogale FM. Biomass-based adsorbents for removal of dyes from wastewater: A review. Front. Environ. Sci. 2021;9:558. doi: 10.3389/fenvs. - DOI
-
- Sureshkumar V, Daniel SK, Ruckmani K, Sivakumar M. Fabrication of chitosan–magnetite nanocomposite strip for chromium removal. Appl. Nanosci. 2016;6(2):277–285. doi: 10.1007/s13204-015-0429-3. - DOI
-
- Mukherjee T, Das P, Ghosh SK, Rahaman M. Removal of Alizarin Red S from Wastewater: Optimizing the Process Parameters for Electrocoagulation Using Taguchi Method. Springer; 2019.
LinkOut - more resources
Full Text Sources

