LncRNA FAS-AS1 upregulated by its genetic variation rs6586163 promotes cell apoptosis in nasopharyngeal carcinoma through regulating mitochondria function and Fas splicing
- PMID: 37217794
- PMCID: PMC10203136
- DOI: 10.1038/s41598-023-35502-z
LncRNA FAS-AS1 upregulated by its genetic variation rs6586163 promotes cell apoptosis in nasopharyngeal carcinoma through regulating mitochondria function and Fas splicing
Abstract
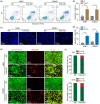
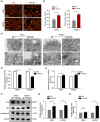
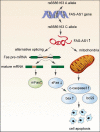
Nasopharyngeal carcinoma (NPC) is a common head and neck malignant with a high incidence in Southern China. Genetic aberrations play a vital role in the pathogenesis, progression and prognosis of NPC. In the present study, we elucidated the underlying mechanism of FAS-AS1 and its genetic variation rs6586163 in NPC. We demonstrated that FAS-AS1 rs6586163 variant genotype carriers were associated with lower risk of NPC (CC vs. AA, OR = 0.645, P = 0.006) and better overall survival (AC + CC vs. AA, HR = 0.667, P = 0.030). Mechanically, rs6586163 increased the transcriptional activity of FAS-AS1 and contributed to ectopic overexpression of FAS-AS1 in NPC. rs6586163 also exhibited an eQTL trait and the genes affected by rs6586163 were enriched in apoptosis related signaling pathway. FAS-AS1 was downregulated in NPC tissues and over-expression of FAS-AS1 was associated with early clinical stage and better short-term treatment efficacy for NPC patients. Overexpression of FAS-AS1 inhibited NPC cell viability and promoted cell apoptosis. GSEA analysis of RNA-seq data suggested FAS-AS1 participate in mitochondria regulation and mRNA alternative splicing. Transmission electron microscopic examination verified that the mitochondria was swelled, the mitochondrial cristae was fragmented or disappeared, and their structures were destroyed in FAS-AS1 overexpressed cells. Furthermore, we identified HSP90AA1, CS, BCL2L1, SOD2 and PPARGC1A as the top 5 hub genes of FAS-AS1 regulated genes involved in mitochondria function. We also proved FAS-AS1 could affect Fas splicing isoform sFas/mFas expression ratio, and apoptotic protein expression, thus leading to increased apoptosis. Our study provided the first evidence that FAS-AS1 and its genetic polymorphism rs6586163 triggered apoptosis in NPC, which might have a potential as new biomarkers for NPC susceptibility and prognosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Long noncoding RNA PXN-AS1-L promotes the malignancy of nasopharyngeal carcinoma cells via upregulation of SAPCD2.Cancer Med. 2019 Aug;8(9):4278-4291. doi: 10.1002/cam4.2227. Epub 2019 Jun 7. Cancer Med. 2019. PMID: 31173488 Free PMC article.
-
Long noncoding RNA PTPRG-AS1 acts as a microRNA-194-3p sponge to regulate radiosensitivity and metastasis of nasopharyngeal carcinoma cells via PRC1.J Cell Physiol. 2019 Aug;234(10):19088-19102. doi: 10.1002/jcp.28547. Epub 2019 Apr 16. J Cell Physiol. 2019. PMID: 30993702
-
Long non-coding RNA PTPRG-AS1/microRNA-124-3p regulates radiosensitivity of nasopharyngeal carcinoma via the LIM Homeobox 2-dependent Notch pathway through competitive endogenous RNA mechanism.Bioengineered. 2022 Apr;13(4):8208-8225. doi: 10.1080/21655979.2022.2037364. Bioengineered. 2022. PMID: 35300558 Free PMC article.
-
lncRNA CASC2/miR‑18a‑5p axis regulates the malignant potential of nasopharyngeal carcinoma by targeting RBBP8.Oncol Rep. 2019 Mar;41(3):1797-1806. doi: 10.3892/or.2018.6941. Epub 2018 Dec 19. Oncol Rep. 2019. PMID: 30569153
-
Mechanism of HOXA10 in nasopharyngeal carcinoma cell proliferation through the PTPRG-AS1/USP1 axis.J Biochem Mol Toxicol. 2024 Nov;38(11):e70025. doi: 10.1002/jbt.70025. J Biochem Mol Toxicol. 2024. PMID: 39445487
Cited by
-
Unveiling the Network regulatory mechanism of ncRNAs on the Ferroptosis Pathway: Implications for Preeclampsia.Int J Womens Health. 2024 Sep 30;16:1633-1651. doi: 10.2147/IJWH.S485653. eCollection 2024. Int J Womens Health. 2024. PMID: 39372667 Free PMC article. Review.
-
Solasodine suppresses nasopharyngeal carcinoma progression by inducing ferroptosis.Sci Rep. 2025 May 18;15(1):17247. doi: 10.1038/s41598-025-93834-4. Sci Rep. 2025. PMID: 40383858 Free PMC article.
-
miRNAs that regulate apoptosis in breast cancer and cervical cancer.Cell Biochem Biophys. 2024 Sep;82(3):1993-2006. doi: 10.1007/s12013-024-01405-7. Epub 2024 Jul 6. Cell Biochem Biophys. 2024. PMID: 38969951 Review.
-
Neuroprotective and vasoprotective effects of herb pair of Zhiqiao-Danggui in ischemic stroke uncovered by LC-MS/MS-based metabolomics approach.Metab Brain Dis. 2024 Aug;39(6):1131-1148. doi: 10.1007/s11011-024-01387-8. Epub 2024 Jul 13. Metab Brain Dis. 2024. PMID: 39002017
-
Improved health outcomes of nasopharyngeal carcinoma patients 3 years after treatment by the AI-assisted home enteral nutrition management.Front Nutr. 2025 Jan 7;11:1481073. doi: 10.3389/fnut.2024.1481073. eCollection 2024. Front Nutr. 2025. PMID: 39839291 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

