S100 proteins in cardiovascular diseases
- PMID: 37217870
- PMCID: PMC10204201
- DOI: 10.1186/s10020-023-00662-1
S100 proteins in cardiovascular diseases
Abstract
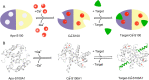
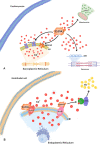
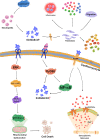
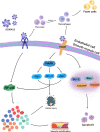
Cardiovascular diseases have become a serious threat to human health and life worldwide and have the highest fatality rate. Therefore, the prevention and treatment of cardiovascular diseases have become a focus for public health experts. The expression of S100 proteins is cell- and tissue-specific; they are implicated in cardiovascular, neurodegenerative, and inflammatory diseases and cancer. This review article discusses the progress in the research on the role of S100 protein family members in cardiovascular diseases. Understanding the mechanisms by which these proteins exert their biological function may provide novel concepts for preventing, treating, and predicting cardiovascular diseases.
Keywords: Cardiovascular diseases; RAGE; S100 proteins; TLR-4.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

