Novel Anti-HER2 Antibody-Drug Conjugates Versus T-DM1 for HER2-Positive Metastatic Breast Cancer After Tyrosine Kinase Inhibitors Treatment
- PMID: 37218076
- PMCID: PMC10546815
- DOI: 10.1093/oncolo/oyad127
Novel Anti-HER2 Antibody-Drug Conjugates Versus T-DM1 for HER2-Positive Metastatic Breast Cancer After Tyrosine Kinase Inhibitors Treatment
Abstract
Background: Antibody-drug conjugates (ADCs) have been the preferred regimens for human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC) after trastuzumab. Unfortunately, there is little data showing which ADCs should be chosen for those patients whose treatment with tyrosine kinase inhibitors (TKIs) failed. This study aims to analyze the efficacy and safety between novel anti-HER2 ADCs and trastuzumab emtansine (T-DM1) for those with TKIs failure.
Materials and methods: HER2-positive MBC using ADCs from January 2013 to June 2022 were included, and all of them were treated with TKIs. The primary study endpoint was progression-free survival (PFS), and the secondary study endpoints were objective response rate (ORR), clinical benefit rate (CBR), and safety.
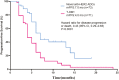
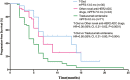
Results: A total of 144 patients with 73 patients in the novel anti-HER2 ADCs group and 71 patients in the T-DM1 group. In these novel ADCs, 30 patients received trastuzumab deruxtecan (T-Dxd), 43 patients receive other novel ADCs. The median PFS in the novel ADCs group and T-DM1 group were 7.0 months versus 4.0 months, respectively, and ORR was 54.8% versus 22.5%, CBR was 65.8% versus 47.9%, respectively. In subgroups analysis, the PFS were both significantly improved in patients receiving T-Dxd and other novel ADCs compared with T-DM1. The most common grades 3-4 adverse events in the novel anti-HER-2 ADCs group were neutropenia (20.5%) and thrombocytopenia (28.1%) in the T-DM1 group.
Conclusions: In patients with HER2-positive MBC previously treated with TKIs, both T-Dxd and other novel anti-HER2 ADCs yielded statistically significant better PFS than T-DM1 did, with tolerable toxicities.
Keywords: antibody-drug conjugates; metastatic breast cancer; trastuzumab deruxtecan; trastuzumab emtansine; tyrosine kinase inhibitor.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
The authors indicated no financial relationships.
Figures

Similar articles
-
Novel anti-HER2 ADCs vs dual anti-HER2 antibody for HER2-positive metastatic breast cancer failed to tyrosine kinase inhibitor.Oncologist. 2025 Jan 17;30(1):oyae144. doi: 10.1093/oncolo/oyae144. Oncologist. 2025. PMID: 39786451 Free PMC article.
-
Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial.ESMO Open. 2024 May;9(5):102924. doi: 10.1016/j.esmoop.2024.102924. Epub 2024 Apr 24. ESMO Open. 2024. PMID: 38796287 Free PMC article. Clinical Trial.
-
Trastuzumab deruxtecan versus trastuzumab emtansine in Asian patients with HER2-positive metastatic breast cancer.Cancer Sci. 2024 Sep;115(9):3079-3088. doi: 10.1111/cas.16234. Epub 2024 Jul 9. Cancer Sci. 2024. PMID: 38979893 Free PMC article. Clinical Trial.
-
Trastuzumab-emtansine versus other anti-HER2 regimens in early or unresectable or metastatic HER-2 positive breast cancer: systematic review and network meta-analysis.Rev Peru Med Exp Salud Publica. 2024 May 27;41(1):7-18. doi: 10.17843/rpmesp.2024.411.13351. Rev Peru Med Exp Salud Publica. 2024. PMID: 38808848 Free PMC article.
-
The evolving therapeutic landscape of trastuzumab-drug conjugates: Future perspectives beyond HER2-positive breast cancer.Cancer Treat Rev. 2023 Feb;113:102500. doi: 10.1016/j.ctrv.2022.102500. Epub 2022 Dec 24. Cancer Treat Rev. 2023. PMID: 36587473 Review.
Cited by
-
Immunoconjugates as an Efficient Platform for Drug Delivery: A Resurgence of Natural Products in Targeted Antitumor Therapy.Pharmaceuticals (Basel). 2024 Dec 17;17(12):1701. doi: 10.3390/ph17121701. Pharmaceuticals (Basel). 2024. PMID: 39770542 Free PMC article. Review.
-
Efficacy of first-line dual oral pyrotinib plus capetabine therapy in HER2-positive metastatic breast cancer: A real-world retrospective study.Cancer Med. 2024 Jun;13(11):e7256. doi: 10.1002/cam4.7256. Cancer Med. 2024. PMID: 38808952 Free PMC article.
-
Novel anti-HER2 ADCs vs dual anti-HER2 antibody for HER2-positive metastatic breast cancer failed to tyrosine kinase inhibitor.Oncologist. 2025 Jan 17;30(1):oyae144. doi: 10.1093/oncolo/oyae144. Oncologist. 2025. PMID: 39786451 Free PMC article.
-
Impact of HER2-targeting antibody drug conjugates in treatment strategies for patients with breast cancer.Heliyon. 2025 Jan 3;11(3):e41590. doi: 10.1016/j.heliyon.2024.e41590. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39916839 Free PMC article. Review.
-
Exploring the Role of ADCs in Brain Metastases and Primary Brain Tumors: Insight and Future Directions.Cancers (Basel). 2025 May 7;17(9):1591. doi: 10.3390/cancers17091591. Cancers (Basel). 2025. PMID: 40361515 Free PMC article. Review.
References
-
- Khongorzul P, Ling CJ, Khan FU, et al. . Antibody-drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18(1):3-19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

