Blockage of Osteopontin-Integrin β3 Signaling in Infrapatellar Fat Pad Attenuates Osteoarthritis in Mice
- PMID: 37218542
- PMCID: PMC10401113
- DOI: 10.1002/advs.202300897
Blockage of Osteopontin-Integrin β3 Signaling in Infrapatellar Fat Pad Attenuates Osteoarthritis in Mice
Abstract
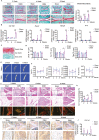
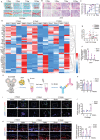
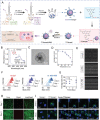
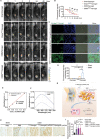
The knowledge of osteoarthritis (OA) has nowadays been extended from a focalized cartilage disorder to a multifactorial disease. Although recent investigations have reported that infrapatellar fat pad (IPFP) can trigger inflammation in the knee joint, the mechanisms behind the role of IPFP on knee OA progression remain to be defined. Here, dysregulated osteopontin (OPN) and integrin β3 signaling are found in the OA specimens of both human and mice. It is further demonstrated that IPFP-derived OPN participates in OA progression, including activated matrix metallopeptidase 9 in chondrocyte hypertrophy and integrin β3 in IPFP fibrosis. Motivated by these findings, an injectable nanogel is fabricated to provide sustained release of siRNA Cd61 (RGD- Nanogel/siRNA Cd61) that targets integrins. The RGD- Nanogel possesses excellent biocompatibility and desired targeting abilities both in vitro and in vivo. Local injection of RGD- Nanogel/siRNA Cd61 robustly alleviates the cartilage degeneration, suppresses the advancement of tidemark, and reduces the subchondral trabecular bone mass in OA mice. Taken together, this study provides an avenue for developing RGD- Nanogel/siRNA Cd61 therapy to mitigate OA progression via blocking OPN-integrin β3 signaling in IPFP.
Keywords: infrapatellar fat pad; integrin β3; nanogel; osteoarthritis; osteopontin; siRNA.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Fat-cartilage axis: the regulation of IL-6/Osteopontin signaling in osteoarthritis of mice.Cell Death Discov. 2025 Jul 15;11(1):325. doi: 10.1038/s41420-025-02622-6. Cell Death Discov. 2025. PMID: 40664639 Free PMC article.
-
miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis.Biomaterials. 2019 Jun;206:87-100. doi: 10.1016/j.biomaterials.2019.03.022. Epub 2019 Mar 20. Biomaterials. 2019. PMID: 30927715
-
The biphasic role of the infrapatellar fat pad in osteoarthritis.Biomed Pharmacother. 2024 Oct;179:117364. doi: 10.1016/j.biopha.2024.117364. Epub 2024 Sep 2. Biomed Pharmacother. 2024. PMID: 39226725 Review.
-
Extracellular Vesicles in Infrapatellar Fat Pad from Osteoarthritis Patients Impair Cartilage Metabolism and Induce Senescence.Adv Sci (Weinh). 2024 Jan;11(3):e2303614. doi: 10.1002/advs.202303614. Epub 2023 Nov 30. Adv Sci (Weinh). 2024. PMID: 38036301 Free PMC article.
-
The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review.Osteoarthritis Cartilage. 2010 Jul;18(7):876-82. doi: 10.1016/j.joca.2010.03.014. Epub 2010 Apr 22. Osteoarthritis Cartilage. 2010. PMID: 20417297 Review.
Cited by
-
Fat-cartilage axis: the regulation of IL-6/Osteopontin signaling in osteoarthritis of mice.Cell Death Discov. 2025 Jul 15;11(1):325. doi: 10.1038/s41420-025-02622-6. Cell Death Discov. 2025. PMID: 40664639 Free PMC article.
-
Thrombospondin-1 mitigates osteoarthritis progression by inhibiting mechanical stress-induced chondrocyte ferroptosis via the integrin/YAP pathway.Front Immunol. 2025 May 22;16:1577234. doi: 10.3389/fimmu.2025.1577234. eCollection 2025. Front Immunol. 2025. PMID: 40475787 Free PMC article.
-
The immune duality of osteopontin and its therapeutic implications for kidney transplantation.Front Immunol. 2025 Feb 28;16:1520777. doi: 10.3389/fimmu.2025.1520777. eCollection 2025. Front Immunol. 2025. PMID: 40093009 Free PMC article. Review.
-
Mussel-inspired cortical bone-adherent bioactive composite hydrogels promote bone augmentation through sequential regulation of endochondral ossification.Mater Today Bio. 2023 Oct 25;23:100843. doi: 10.1016/j.mtbio.2023.100843. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 37942424 Free PMC article.
-
Immunoregulatory Roles of Osteopontin in Diseases.Nutrients. 2024 Jan 20;16(2):312. doi: 10.3390/nu16020312. Nutrients. 2024. PMID: 38276550 Free PMC article. Review.
References
-
- Martel‐Pelletier J., Barr A. J., Cicuttini F. M., Conaghan P. G., Cooper C., Goldring M. B., Goldring S. R., Jones G., Teichtahl A. J., Pelletier J.‐P., Nat. Rev. Dis. Primers 2016, 2, 16072. - PubMed
-
- van denBerg W. B., Osteoarthritis Cartilage 1999, 7, 333. - PubMed
-
- Ruhdorfer A., Wirth W., Eckstein F., Arthritis Rheumatol 2016, 68, 826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
