Rapid Detection of DNA and RNA Shrimp Viruses Using CRISPR-Based Diagnostics
- PMID: 37219435
- PMCID: PMC10304985
- DOI: 10.1128/aem.02151-22
Rapid Detection of DNA and RNA Shrimp Viruses Using CRISPR-Based Diagnostics
Abstract
Timely detection of persistent and emerging pathogens is critical to controlling disease spread, particularly in high-density populations with increased contact between individuals and limited-to-no ability to quarantine. Standard molecular diagnostic tests for surveying pathogenic microbes have provided the sensitivity needed for early detection, but lag in time-to-result leading to delayed action. On-site diagnostics alleviate this lag, but current technologies are less sensitive and adaptable than lab-based molecular methods. Towards the development of improved on-site diagnostics, we demonstrated the adaptability of a loop-mediated isothermal amplification-CRISPR coupled technology for detecting DNA and RNA viruses that have greatly impacted shrimp populations worldwide; White Spot Syndrome Virus and Taura Syndrome Virus. Both CRISPR-based fluorescent assays we developed showed similar sensitivity and accuracy for viral detection and load quantification to real-time PCR. Additionally, both assays specifically targeted their respective virus with no false positives detected in animals infected with other common pathogens or in certified specific pathogen-free animals. IMPORTANCE The Pacific white shrimp (Penaeus vannamei) is one of the most valuable aquaculture species in the world but has suffered major economic losses from outbreaks of White Spot Syndrome Virus and Taura Syndrome Virus. Rapid detection of these viruses can improve aquaculture practices by enabling more timely action to be taken to combat disease outbreaks. Highly sensitive, specific, and robust CRISPR-based diagnostic assays such as those developed here have the potential to revolutionize disease management in agriculture and aquaculture helping to promote global food security.
Keywords: assay development; diagnostics; pathogens; rapid tests.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
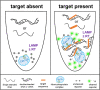

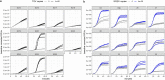
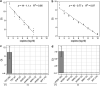

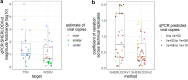
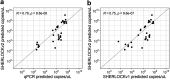
References
-
- Warke D. 2022. CRISPR-based tools: alternative methods for diagnosis of diseases. The Pharma Innovation J 11:1891–1896.
-
- Cunha MV, Inácio J. 2015. Overview and challenges of molecular technologies in the veterinary microbiology laboratory. In Cunha M, Inácio J (eds), Veterinary infection biology: molecular diagnostics and high-throughput strategies. Methods in Molecular Biology, vol 1247. Humana Press, New York, NY. doi:10.1007/978-1-4939-2004-4_1. - DOI - PubMed
-
- Jayamohan H, Lambert CJ, Sant HJ, Jafek A, Patel D, Feng H, Beeman M, Mahmood T, Nze U, Gale BK. 2021. SARS-CoV-2 pandemic: a review of molecular diagnostic tools including sample collection and commercial response with associated advantages and limitations. Anal Bioanal Chem 413:49–71. doi:10.1007/s00216-020-02958-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

