Hairy gene homolog increases nasopharyngeal carcinoma cell stemness by upregulating Bmi-1
- PMID: 37219449
- PMCID: PMC10258013
- DOI: 10.18632/aging.204742
Hairy gene homolog increases nasopharyngeal carcinoma cell stemness by upregulating Bmi-1
Abstract
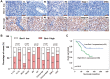
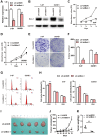
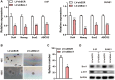
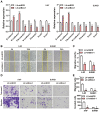
B-cell-specific Moloney murine leukemia virus integration site 1 (Bmi-1) is overexpressed in various cancer types. We found that Bmi-1 mRNA levels were elevated in nasopharyngeal carcinoma (NPC) cell lines. In immunohistochemical analyses, high Bmi-1 levels were observed in not only 5 of 38 non-cancerous nasopharyngeal squamous epithelial biopsies, but also in 66 of 98 NPC specimens (67.3%). High Bmi-1 levels were detected more frequently in T3-T4, N2-N3 and stage III-IV NPC biopsies than in T1-T2, N0-N1 and stage I-II NPC samples, indicating that Bmi-1 is upregulated in advanced NPC. In 5-8F and SUNE1 NPC cells, stable depletion of Bmi-1 using lentiviral RNA interference greatly suppressed cell proliferation, induced G1-phase cell cycle arrest, reduced cell stemness and suppressed cell migration and invasion. Likewise, knocking down Bmi-1 inhibited NPC cell growth in nude mice. Both chromatin immunoprecipitation and Western blotting assays demonstrated that Hairy gene homolog (HRY) upregulated Bmi-1 by binding to its promoter, thereby increasing the stemness of NPC cells. Immunohistochemistry and quantitative real-time PCR analyses revealed that HRY expression correlated positively with Bmi-1 expression in a cohort of NPC biopsies. These findings suggested that HRY promotes NPC cell stemness by upregulating Bmi-1, and that silencing Bmi-1 can suppress NPC progression.
Keywords: Bmi-1; HRY; cell proliferation; migration; nasopharyngeal carcinoma (NPC).
Conflict of interest statement
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

