Apigenin Alleviates Autoimmune Uveitis by Inhibiting Microglia M1 Pro-Inflammatory Polarization
- PMID: 37219511
- PMCID: PMC10210511
- DOI: 10.1167/iovs.64.5.21
Apigenin Alleviates Autoimmune Uveitis by Inhibiting Microglia M1 Pro-Inflammatory Polarization
Abstract
Purpose: Apigenin is a natural small molecule compound widely present in various vegetables and fruits. Recently, Apigenin was reported to inhibit lipopolysaccharide (LPS)-simulated microglial proinflammatory activation. Considering the important role of microglia in retinal disorders, we wonder whether Apigenin could exert a therapeutic effect on experimental autoimmune uveitis (EAU) through reprogramming retinal microglia to a beneficial subtype.
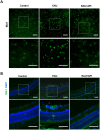
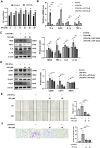
Methods: EAU was induced in C57BL/6J mice by immunization with interphotoreceptor retinoid-binding protein (IRBP)651-670, followed by intraperitoneal administration of Apigenin. Disease severity was assessed based on clinical and pathological scores. In vivo, Western blotting was used to quantify protein levels of classical inflammatory factors, microglial M1/M2 markers and the tight junction protein of the blood-retinal-barrier (BRB). Immunofluorescence was used to determine the Apigenin's efficacy on microglial phenotype. In vitro, Apigenin was added in LPS and IFN-γ stimulated human microglial cell line. Western blotting and Transwell assays were used to analyze the phenotype of microglia.
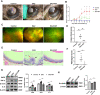
Results: In vivo, we found that Apigenin significantly reduced the clinical and pathological scores of EAU. The protein levels of inflammatory cytokines were significantly decreased in retina, and BRB disruption was ameliorated after Apigenin treatment. Meanwhile, Apigenin inhibited microglia M1 transition in EAU mice retina. In vitro functional studies showed that Apigenin decreased LPS and IFN-γ-induced microglial inflammatory factor production and M1-activation via the TLR4/MyD88 pathway.
Conclusions: Apigenin can ameliorate retinal inflammation in IRBP induced autoimmune uveitis through inhibiting microglia M1 pro-inflammatory polarization via TLR4/MyD88 pathway.
Conflict of interest statement
Disclosure:
Figures


Similar articles
-
Galectin-3 regulates microglial activation and promotes inflammation through TLR4/MyD88/NF-kB in experimental autoimmune uveitis.Clin Immunol. 2022 Mar;236:108939. doi: 10.1016/j.clim.2022.108939. Epub 2022 Feb 1. Clin Immunol. 2022. PMID: 35121106
-
Aryl Hydrocarbon Receptor Regulates Apoptosis and Inflammation in a Murine Model of Experimental Autoimmune Uveitis.Front Immunol. 2018 Jul 25;9:1713. doi: 10.3389/fimmu.2018.01713. eCollection 2018. Front Immunol. 2018. PMID: 30090104 Free PMC article.
-
Isovitexin-Mediated Regulation of Microglial Polarization in Lipopolysaccharide-Induced Neuroinflammation via Activation of the CaMKKβ/AMPK-PGC-1α Signaling Axis.Front Immunol. 2019 Nov 14;10:2650. doi: 10.3389/fimmu.2019.02650. eCollection 2019. Front Immunol. 2019. PMID: 31798583 Free PMC article.
-
Aminooxy-Acetic Acid Inhibits Experimental Autoimmune Uveitis by Modulating the Balance between Effector and Regulatory Lymphocyte Subsets.Curr Mol Med. 2020;20(8):624-632. doi: 10.2174/1566524020666200211112219. Curr Mol Med. 2020. PMID: 32072910
-
Microglial-mediated immune mechanisms in autoimmune uveitis: Elucidating pathogenic pathways and targeted therapeutics.J Neuroimmunol. 2024 Oct 15;395:578433. doi: 10.1016/j.jneuroim.2024.578433. Epub 2024 Aug 14. J Neuroimmunol. 2024. PMID: 39168018 Review.
Cited by
-
Targeting Senescence, Oxidative Stress, and Inflammation: Quercetin-Based Strategies for Ocular Diseases in Older Adults.Clin Interv Aging. 2025 Jun 7;20:791-813. doi: 10.2147/CIA.S516946. eCollection 2025. Clin Interv Aging. 2025. PMID: 40503074 Free PMC article. Review.
-
Transcription factor EGR2 alleviates autoimmune uveitis via activation of GDF15 to modulate the retinal microglial phenotype.Proc Natl Acad Sci U S A. 2024 Sep 24;121(39):e2316161121. doi: 10.1073/pnas.2316161121. Epub 2024 Sep 19. Proc Natl Acad Sci U S A. 2024. PMID: 39298490 Free PMC article.
-
Research progress on the regulatory and pharmacological mechanism of chemical components of Dendrobium.Heliyon. 2024 Sep 7;10(18):e37541. doi: 10.1016/j.heliyon.2024.e37541. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39328574 Free PMC article. Review.
-
Single‐cell RNA sequencing reveals inflammatory retinal microglia in experimental autoimmune uveitis.MedComm (2020). 2024 Apr 7;5(4):e534. doi: 10.1002/mco2.534. eCollection 2024 Apr. MedComm (2020). 2024. PMID: 38585235 Free PMC article. Italian.
-
Muscone Attenuates Uveitis Through the PI3K/AKT Signaling Pathway.Invest Ophthalmol Vis Sci. 2025 May 1;66(5):21. doi: 10.1167/iovs.66.5.21. Invest Ophthalmol Vis Sci. 2025. PMID: 40341311 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

