Escaping the enemy's bullets: an update on how malaria parasites evade host immune response
- PMID: 37219610
- PMCID: PMC10348937
- DOI: 10.1007/s00436-023-07868-6
Escaping the enemy's bullets: an update on how malaria parasites evade host immune response
Abstract
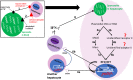
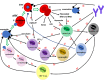
Malaria continues to cause untold hardship to inhabitants of malaria-endemic regions, causing significant morbidity and mortality that severely impact global health and the economy. Considering the complex life cycle of malaria parasites (MPs) and malaria biology, continued research efforts are ongoing to improve our understanding of the pathogenesis of the diseases. Female Anopheles mosquito injects MPs into its hosts during a blood meal, and MPs invade the host skin and the hepatocytes without causing any serious symptoms. Symptomatic infections occur only during the erythrocytic stage. In most cases, the host's innate immunity (for malaria-naïve individuals) and adaptive immunity (for pre-exposed individuals) mount severe attacks and destroy most MPs. It is increasingly understood that MPs have developed several mechanisms to escape from the host's immune destruction. This review presents recent knowledge on how the host's immune system destroys invading MPs as well as MPs survival or host immune evasion mechanisms. On the invasion of host cells, MPs release molecules that bind to cell surface receptors to reprogram the host in a way to lose the capacity to destroy them. MPs also hide from the host immune cells by inducing the clustering of both infected and uninfected erythrocytes (rosettes), as well as inducing endothelial activation. We hope this review will inspire more research to provide a complete understanding of malaria biology and promote interventions to eradicate the notorious disease.
Keywords: Evasion of host immunity; Host immune response; Malaria; Plasmodium infection; Rosetting.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors declare no competing interests.
Figures
Similar articles
-
Additional Feeding Reveals Differences in Immune Recognition and Growth of Plasmodium Parasites in the Mosquito Host.mSphere. 2021 Mar 31;6(2):e00136-21. doi: 10.1128/mSphere.00136-21. mSphere. 2021. PMID: 33789941 Free PMC article.
-
Host immune evasion strategies of malaria blood stage parasite.Mol Biosyst. 2017 Nov 21;13(12):2498-2508. doi: 10.1039/c7mb00502d. Mol Biosyst. 2017. PMID: 29091093 Review.
-
Lessons Learned for Pathogenesis, Immunology, and Disease of Erythrocytic Parasites: Plasmodium and Babesia.Front Cell Infect Microbiol. 2021 Aug 3;11:685239. doi: 10.3389/fcimb.2021.685239. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34414129 Free PMC article. Review.
-
Immune Response and Evasion Mechanisms of Plasmodium falciparum Parasites.J Immunol Res. 2018 Mar 25;2018:6529681. doi: 10.1155/2018/6529681. eCollection 2018. J Immunol Res. 2018. PMID: 29765991 Free PMC article. Review.
-
Molecular basis for evasion of host immunity and pathogenesis in malaria.Biochim Biophys Acta. 1998 Feb 27;1406(1):10-27. doi: 10.1016/s0925-4439(97)00078-1. Biochim Biophys Acta. 1998. PMID: 9545516 Review.
Cited by
-
Time-of-day of infection: impact on liver stage malaria parasites in untreated and drug-treated hosts.Parasit Vectors. 2025 Aug 6;18(1):339. doi: 10.1186/s13071-025-06986-7. Parasit Vectors. 2025. PMID: 40770720 Free PMC article.
-
Dynamics of osteopontin levels and correlation with parasitemia in acute malaria in Uganda and Sweden.BMC Infect Dis. 2024 Oct 15;24(1):1164. doi: 10.1186/s12879-024-10076-x. BMC Infect Dis. 2024. PMID: 39407132 Free PMC article.
-
Plant-derived nanomaterials (PDNM): a review on pharmacological potentials against pathogenic microbes, antimicrobial resistance (AMR) and some metabolic diseases.3 Biotech. 2023 Sep;13(9):291. doi: 10.1007/s13205-023-03713-w. Epub 2023 Aug 4. 3 Biotech. 2023. PMID: 37547919 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

