Correlations between three ELISA protocols measurements of RTS,S/AS01-induced anti-CSP IgG antibodies
- PMID: 37220123
- PMCID: PMC10204981
- DOI: 10.1371/journal.pone.0286117
Correlations between three ELISA protocols measurements of RTS,S/AS01-induced anti-CSP IgG antibodies
Abstract
Background: RTS,S/AS01 induced anti-circumsporozoite protein (CSP) IgG antibodies are associated with the vaccine efficacy. There is currently no international standardisation of the assays used in the measurement of anti-CSP IgG antibody concentrations for use in evaluations of the vaccine's immunogenicity and/or efficacy. Here, we compared the levels of RTS,S/AS01 induced anti-CSP IgG antibodies measured using three different enzyme-Linked ImmunoSorbent Assays (ELISA).
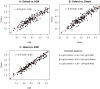
Methods: 196 plasma samples were randomly selected from the 447 samples collected during the RTS,S/AS01 phase IIb trial in 2007 from Kenyan children aged between 5-17 months. The vaccine-induced anti-CSP IgG antibodies were then measured using two independently developed ELISA protocols ('Kilifi-RTS,S' and 'Oxford-R21') and compared to the results from the reference 'Ghent-RTS,S' protocol for the same participants. For each pair of protocols, a deming regression model was fitted. Linear equations were then derived to aid in conversions into equivalent ELISA units. The agreement was assessed using Bland and Altman method.
Findings: The anti-CSP IgG antibodies measured from the three ELISA protocols were in agreement, and were positively and linearly correlated; 'Oxford' and 'Kilifi' r = 0.93 (95% CI 0.91-0.95), 'Oxford' and 'Ghent' r = 0.94 (95% CI: 0.92-0.96), and 'Kilifi' and 'Ghent' r = 0.97 (95% CI: 0.96-0.98), p<0.0001 for all correlations.
Conclusions: With the linearity, agreement and correlations established between the assays, conversion equations can be applied to convert results into equivalent units, enabling comparisons of immunogenicities across different vaccines of the same CSP antigens. This study highlights the need for the international harmonisation of anti-CSP antibody measurements.
Copyright: © 2023 Mugo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization (WHO). World malaria report 2022.
-
- WHO recommends groundbreaking malaria vaccine for children at risk. Geneva, World Health Organization. 2021. https://www.who.int/news/item/06-10-2021.
-
- Datoo MS, Natama MH, Somé A, Traoré O, Rouamba T, Bellamy D, et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. The Lancet. 2021. doi: 10.1016/S0140-6736(21)00943-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

