Cardiovascular and Renal Benefits of Novel Diabetes Drugs by Baseline Cardiovascular Risk: A Systematic Review, Meta-analysis, and Meta-regression
- PMID: 37220263
- PMCID: PMC10234755
- DOI: 10.2337/dc22-0772
Cardiovascular and Renal Benefits of Novel Diabetes Drugs by Baseline Cardiovascular Risk: A Systematic Review, Meta-analysis, and Meta-regression
Abstract
Background: Eligibility for glucagon-like peptide 1 receptor agonists (GLP-1RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i) has been expanded to patients with diabetes at lower cardiovascular risk, but whether treatment benefits differ by risk levels is not clear.
Purpose: To investigate whether patients with varying risks differ in cardiovascular and renal benefits from GLP-1RA and SGLT2i with use of meta-analysis and meta-regression.
Data sources: We performed a systematic review using PubMed through 7 November 2022.
Study selection: We included reports of GLP-1RA and SGLT2i confirmatory randomized trials in adult patients with safety or efficacy end point data.
Data extraction: Hazard ratio (HR) and event rate data were extracted for mortality, cardiovascular, and renal outcomes.
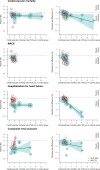
Data synthesis: We analyzed 9 GLP-1RA and 13 SGLT2i trials comprising 154,649 patients. Summary HRs were significant for cardiovascular mortality (GLP-1RA 0.87 and SGLT2i 0.86), major adverse cardiovascular events (0.87 and 0.88), heart failure (0.89 and 0.70), and renal (0.84 and 0.65) outcomes. For stroke, efficacy was significant for GLP-1RA (0.84) but not for SGLT2i (0.92). Associations between control arm cardiovascular mortality rates and HRs were nonsignificant. Five-year absolute risk reductions (0.80-4.25%) increased to 11.6% for heart failure in SGLT2i trials in patients with high risk (Pslope < 0.001). For GLP1-RAs, associations were nonsignificant.
Limitations: Analyses were limited by lack of patient-level data, consistency in end point definitions, and variation in cardiovascular mortality rates for GLP-1RA trials.
Conclusions: Relative effects of novel diabetes drugs are preserved across baseline cardiovascular risk, whereas absolute benefits increase at higher risks, particularly regarding heart failure. Our findings suggest a need for baseline risk assessment tools to identify variation in absolute treatment benefits and improve decision-making.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures



Comment in
-
Absolute Benefits From Glucagon-Like Peptide 1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors.Diabetes Care. 2023 Jun 1;46(6):1143-1144. doi: 10.2337/dci23-0023. Diabetes Care. 2023. PMID: 37220264 Free PMC article. No abstract available.
References
-
- International Diabetes Federation . IDF Diabetes Atlas. Brussels, Belgium, International Diabetes Federation, 2021
-
- Alva ML, Hoerger TJ, Zhang P, Cheng YJ. State-level diabetes-attributable mortality and years of life lost in the United States. Ann Epidemiol 2018;28:790–795 - PubMed
-
- United States Renal Data System . USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 2021
-
- Sattar N, Lee MMY, Kristensen SL, et al. . Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–662 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

