Separation and Detection of Tyrosine and Phenylalanine-derived Oxidative Stress Biomarkers Using Microchip Electrophoresis with Electrochemical Detection
- PMID: 37220612
- PMCID: PMC10202003
- DOI: 10.1002/elan.202100580
Separation and Detection of Tyrosine and Phenylalanine-derived Oxidative Stress Biomarkers Using Microchip Electrophoresis with Electrochemical Detection
Abstract
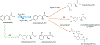
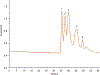
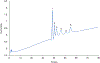
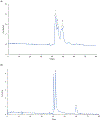
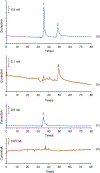
A method for the determination of selected aromatic amino acid biomarkers of oxidative stress using microchip electrophoresis with electrochemical detection is described. The separation of the major reaction products of phenylalanine and tyrosine with reactive nitrogen and oxygen species was accomplished using ligand exchange micellar electrokinetic chromatography with a PDMS/glass hybrid chip. Electrochemical detection was achieved using a pyrolyzed photoresist film working electrode. The system was evaluated for the analysis of the products of the Fenton reaction with tyrosine and phenylalanine, and the reaction of peroxynitrite with tyrosine.
Keywords: hydroxyl radical; microchip electrophoresis with electrochemical detection; peroxynitrite; reactive nitrogen and oxygen species; tyrosine.
Figures



Similar articles
-
Microchip electrophoresis with electrochemical detection for the determination of analytes in the dopamine metabolic pathway.Electrophoresis. 2015 Aug;36(16):1912-9. doi: 10.1002/elps.201500150. Epub 2015 Jun 29. Electrophoresis. 2015. PMID: 25958983 Free PMC article.
-
Aromatic hydroxylation and nitration of phenylalanine and tyrosine by peroxynitrite. Evidence for hydroxyl radical production from peroxynitrite.FEBS Lett. 1994 Feb 14;339(1-2):89-92. doi: 10.1016/0014-5793(94)80391-9. FEBS Lett. 1994. PMID: 8313984
-
Trace analysis of D-tyrosine in biological samples by microchip electrophoresis with laser induced fluorescence detection.J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Nov 1;879(29):3203-7. doi: 10.1016/j.jchromb.2011.01.033. Epub 2011 Feb 4. J Chromatogr B Analyt Technol Biomed Life Sci. 2011. PMID: 21342793
-
[Laboratory on a microfluidic chip].Se Pu. 2005 Sep;23(5):456-63. Se Pu. 2005. PMID: 16350786 Review. Chinese.
-
Recent developments in electrochemical detection for microchip capillary electrophoresis.Electrophoresis. 2004 Nov;25(21-22):3528-49. doi: 10.1002/elps.200406115. Electrophoresis. 2004. PMID: 15565707 Review.
References
-
- Ozcan A, Ogun M in Basic principles and clinical significance of oxidative stress,1st ed., Vol.3 (Ed.:Gowder SJT), IntechOPen, Croatia, 2015, 37–58.
-
- Lichtenberg D, Pinchuk I, Biochem. Biophys. Res. Commun 2015, 461, 441–444. - PubMed
-
- Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI, Eur. J. Med. Chem 2020, 112891. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
