Changes in Inflammatory Markers in Patients Treated for Buruli Ulcer and Their Ability to Predict Paradoxical Reactions
- PMID: 37221015
- PMCID: PMC10681857
- DOI: 10.1093/infdis/jiad176
Changes in Inflammatory Markers in Patients Treated for Buruli Ulcer and Their Ability to Predict Paradoxical Reactions
Abstract
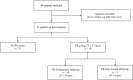
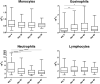
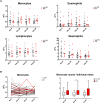
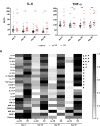
Mycobacterium ulcerans causes Buruli ulcer, the third most frequent mycobacterial disease after tuberculosis and leprosy. Transient clinical deteriorations, known as paradoxical reactions (PRs), occur in some patients during or after antibiotic treatment. We investigated the clinical and biological features of PRs in a prospective cohort of 41 patients with Buruli ulcer from Benin. Neutrophil counts decreased from baseline to day 90, and interleukin 6 (IL-6), granulocyte colony-stimulating factor, and vascular endothelial growth factor were the cytokines displaying a significant monthly decrease relative to baseline. PRs occurred in 10 (24%) patients. The baseline biological and clinical characteristics of the patients presenting with PRs did not differ significantly from those of the other patients. However, the patients with PRs had significantly higher IL-6 and tumor necrosis factor alpha (TNF-α) concentrations on days 30, 60, and 90 after the start of antibiotic treatment. The absence of a decrease in IL-6 and TNF-α levels during treatment should alert clinicians to the possibility of PR onset.
Keywords: Mycobacterium ulcerans; Buruli ulcer; inflammatory response; paradoxical reaction.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Chauty A, Ardant MF, Marsollier L, et al. Oral treatment for Mycobacterium ulcerans infection: results from a pilot study in Benin. Clin Infect Dis 2011; 52:94–6. - PubMed
-
- Barogui Y, Johnson RC, van der Werf TS, et al. Functional limitations after surgical or antibiotic treatment for Buruli ulcer in Benin. Am J Trop Med Hyg 2009; 81:82–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

