Serum neurofilament light chain levels at attack predict post-attack disability worsening and are mitigated by inebilizumab: analysis of four potential biomarkers in neuromyelitis optica spectrum disorder
- PMID: 37221052
- PMCID: PMC10447388
- DOI: 10.1136/jnnp-2022-330412
Serum neurofilament light chain levels at attack predict post-attack disability worsening and are mitigated by inebilizumab: analysis of four potential biomarkers in neuromyelitis optica spectrum disorder
Abstract
Objective: To investigate relationships between serum neurofilament light chain (sNfL), ubiquitin C-terminal hydrolase L1 (sUCHL1), tau (sTau) and glial fibrillary acidic protein (sGFAP) levels and disease activity/disability in neuromyelitis optica spectrum disorder (NMOSD), and the effects of inebilizumab on these biomarkers in N-MOmentum.
Methods: N-MOmentum randomised participants to receive inebilizumab or placebo with a randomised controlled period (RCP) of 28 weeks and an open-label follow-up period of ≥2 years. The sNfL, sUCHL1, sTau and sGFAP were measured using single-molecule arrays in 1260 scheduled and attack-related samples from N-MOmentum participants (immunoglobulin G (IgG) autoantibodies to aquaporin-4-positive, myelin oligodendrocyte glycoprotein-IgG-positive or double autoantibody-negative) and two control groups (healthy donors and patients with relapsing-remitting multiple sclerosis).
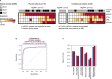
Results: The concentration of all four biomarkers increased during NMOSD attacks. At attack, sNfL had the strongest correlation with disability worsening during attacks (Spearman R2=0.40; p=0.01) and prediction of disability worsening after attacks (sNfL cut-off 32 pg/mL; area under the curve 0.71 (95% CI 0.51 to 0.89); p=0.02), but only sGFAP predicted upcoming attacks. At RCP end, fewer inebilizumab-treated than placebo-treated participants had sNfL>16 pg/mL (22% vs 45%; OR 0.36 (95% CI 0.17 to 0.76); p=0.004).
Conclusions: Compared with sGFAP, sTau and sUCHL1, sNfL at attack was the strongest predictor of disability worsening at attack and follow-up, suggesting a role for identifying participants with NMOSD at risk of limited post-relapse recovery. Treatment with inebilizumab was associated with lower levels of sGFAP and sNfL than placebo.
Trial registration number: NCT02200770.
Keywords: CLINICAL NEUROLOGY; RANDOMISED TRIALS.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: OA reports grants from the German Ministry of Education and Research (BMBF) and the German Research Foundation (DFG); grants and personal fees from Biogen and Novartis; and travel support and personal fees from Alexion, Almirall, MedImmune, Merck Serono, Roche, Sanofi, Viela Bio/Horizon Therapeutics and Zambon. OA is a member of the European Reference Network for Rare Eye Diseases (ERN-EYE), co-funded by the Health Program of the European Union under the Framework Partnership Agreement No 739534 'ERN-EYE. H-PH has received fees for consulting, speaking and serving on steering committees from Bayer Healthcare, Biogen Idec, Celgene Receptos, CSL Behring, GeNeuro, Genzyme, MedDay, MedImmune, Merck Serono, Novartis, Roche, Sanofi, TG Therapeutics and Viela Bio/Horizon Therapeutics, with approval by the Rector of Heinrich Heine University Düsseldorf. KF has received fees for consulting, speaking and serving on steering committees from AbbVie, Alexion, Asahi Kasei Medical, Biogen, Chugai/Roche, Eisai, Japan Tobacco, MedImmune/Viela Bio, Merck, Merck Biopharma, Mitsubishi-Tanabe, Novartis, Takeda, Teijin and UCB, and has received Grant-in-Aid for Scientific Research from the Ministry of Health, Welfare and Labor of Japan. FP has received research support, speaker fees and travel reimbursement from Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme and Teva; is supported by the German Competence Network for Multiple Sclerosis and the German Research Council (DFG Exc 257); has received travel reimbursement from the Guthy–Jackson Charitable Foundation; and serves on the steering committee of the OCTIMS study sponsored by Novartis. RM serves on scientific advisory boards for Alexion, Roche and Viela Bio/Horizon Therapeutics; and has received funding for travel and fees from Alexion, Biogen, Merck, Novartis, Roche and Viela Bio/Horizon Therapeutics. JLB reports payment for study design/consultation from MedImmune/Viela Bio/Horizon Therapeutics; reports personal fees from AbbVie, Alexion, Beigene, Chugai, Clene Nanomedicine, Genentech, Genzyme, Mitsubishi Tanabe Pharma, Reistone Biopharma and Roche; reports grants and personal fees from EMD Serono and Novartis; reports grants from Alexion, Mallinckrodt and the National Institutes of Health; and has a patent for Aquaporumab issued. HJK has received a grant from the National Research Foundation of Korea; consultancy/speaker fees or research support from Alexion, AprilBio, ALTOS Biologics, Biogen, Celltrion, Daewoong, Eisai, GC Pharma, HanAll BioPharma, Handok, Horizon Therapeutics (formerly Viela Bio), Kolon Life Science, MDimune, Mitsubishi Tanabe Pharma, Merck Serono, Novartis, Roche, Sanofi Genzyme, Teva-Handok and UCB; and is a co-editor for the Multiple Sclerosis Journal and an associated editor for the Journal of Clinical Neurology. BGW receives payments for serving as chair of attack adjudication committees for clinical trials in NMOSD for Alexion, MedImmune, UCB Bioscience and Viela Bio/Horizon Therapeutics; has consulted with Chugai, Genentech, Horizon Pharmaceuticals, Mitsubishi Tanabe Pharma and Roche; has received payments for speaking for Genentech and Roche; and has a patent for NMO-IgG for diagnosis of neuromyelitis optica, with royalties paid by Hospices Civils de Lyon, MVZ Labor PD Dr Volkmann und Kollegen GbR, RSR and the University of Oxford. SJP has received personal compensation for serving as a consultant for Astellas, Genentech and Sage Therapeutics; has received personal compensation for serving on scientific advisory boards or data safety monitoring boards for F. Hoffman-La Roche AG, Genentech and UCB; has received research support from Alexion, Roche/Genentech and Viela Bio/MedImmune/Horizon; has a Patent# 8,889,102 (Application#12-678350, Neuromyelitis Optica Autoantibodies as a Marker for Neoplasia)—issued; has a patent, Patent# 9,891,219B2 (Application#12-573942, Methods for Treating Neuromyelitis Optica [NMO] by Administration of Eculizumab to an individual that is Aquaporin-4 (AQP4)-IgG Autoantibody positive)—issued; and his institution has received compensation for serving as a consultant for Alexion, Astellas and Viela Bio/MedImmune/Horizon. DMW reports personal fees from Biogen, Genentech, Horizon, Mitsubishi Tanabe, Roche, UCB Pharma and Viela Bio; and research support paid to Mayo Clinic by Alexion Pharmaceuticals. GC has received personal fees for participation on data and safety monitoring boards from AI Therapeutics, AMO Pharma, AstraZeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Green Valley Pharma, Horizon Pharmaceuticals, Immunic, Karuna Therapeutics, Mapi Pharmaceuticals, Merck, Mitsubishi Tanabe Pharma Holdings, NHLBI (Protocol Review Committee), Novartis, Opko Biologics, Prothena Biosciences, Regeneron, Sanofi-Aventis, Reata Pharmaceuticals, University of Texas Southwestern, University of Pennsylvania and Visioneering Technologies; has received personal fees for consulting or advisory board participation from Alexion, Antisense Therapeutics, Biogen, Clinical Trial Solutions, Entelexo Biotherapeutics, Genentech, Genzyme, GW Pharmaceuticals, Immunic, Klein-Buendel Incorporated, Merck/Serono, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Protalix Biotherapeutics, Recursion/Cerexis Pharmaceuticals, Regeneron, Roche, SAB Biotherapeutics; and is employed by the University of Alabama at Birmingham and President of Pythagoras, a private consulting company located in Birmingham, Alabama. BAC reports personal compensation for consulting from Alexion, Atara Biotherapeutics, Autobahn, Avotres, Biogen, EMD Serono, Gossamer Bio, Horizon, Neuron23, Novartis, Sanofi, TG Therapeutics and Therini Bio; and has received research support from Genentech. MAS, WAR, DS and DC are employees of Horizon Therapeutics and own stock. MG and EK are former employees of Horizon Therapeutics and own stock.
Figures




References
-
- Fujihara K, Misu T, Nakashima I, et al. Neuromyelitis optica should be classified as an astrocytopathic disease rather than a demyelinating disease. Clin Exp Neuroimmunol 2012;3:58–73. 10.1111/j.1759-1961.2012.00030.x Available: http://doi.wiley.com/10.1111/cen3.2012.3.issue-2 - DOI
