Whey-based diet containing medium chain triglycerides modulates the gut microbiota and protects the intestinal mucosa from chemotherapy while maintaining therapy efficacy
- PMID: 37221162
- PMCID: PMC10206084
- DOI: 10.1038/s41419-023-05850-9
Whey-based diet containing medium chain triglycerides modulates the gut microbiota and protects the intestinal mucosa from chemotherapy while maintaining therapy efficacy
Abstract
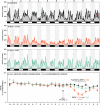
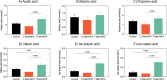
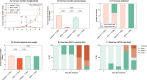
Cytotoxicity (i.e. cell death) is the core mechanism by which chemotherapy induces its anti-cancer effects. Unfortunately, this same mechanism underpins the collateral damage it causes to healthy tissues. The gastrointestinal tract is highly susceptible to chemotherapy's cytotoxicity, resulting in ulcerative lesions (termed gastrointestinal mucositis, GI-M) that impair the functional capacity of the gut leading to diarrhea, anorexia, malnutrition and weight loss, which negatively impact physical/psychological wellbeing and treatment adherence. Preventing these side effects has proven challenging given the overlapping mechanisms that dictate chemotherapy efficacy and toxicity. Here, we report on a novel dietary intervention that, due to its localized gastrointestinal effects, is able to protect the intestinal mucosal from unwanted toxicity without impairing the anti-tumor effects of chemotherapy. The test diet (containing extensively hydrolyzed whey protein and medium chain triglycerides (MCTs)), was investigated in both tumor-naïve and tumor-bearing models to evaluate its effect on GI-M and chemo-efficacy, respectively. In both models, methotrexate was used as the representative chemotherapeutic agent and the diet was provided ad libitum for 14 days prior to treatment. GI-M was measured using the validated biomarker plasma citrulline, and chemo-efficacy defined by tumor burden (cm3/g body weight). The test diet significantly attenuated GI-M (P = 0.03), with associated reductions in diarrhea (P < 0.0001), weight loss (P < 0.05), daily activity (P < 0.02) and maintenance of body composition (P < 0.02). Moreover, the test diet showed significant impact on gut microbiota by increasing diversity and resilience, whilst also altering microbial composition and function (indicated by cecal short and brained chain fatty acids). The test diet did not impair the efficacy of methotrexate against mammary adenocarcinoma (tumor) cells. In line with the first model, the test diet minimized intestinal injury (P = 0.001) and diarrhea (P < 0.0001). These data support translational initiatives to determine the clinical feasibility, utility and efficacy of this diet to improve chemotherapy treatment outcomes.
© 2023. The Author(s).
Conflict of interest statement
All authors have read the journal’s conflicts of interest policy. Dietary products used in this project were provided by Danone Nutricia Research (JvB, MvD, BD, HK, JK). All work presented in this manuscript was conducted independently of Danone Nutricia Research at the University Medical Centre Groningen and the University of Adelaide. All other authors declare no competing interests.
Figures



References
-
- Kuderer NM, Desai A, Lustbery MB, Lyman GH. Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat Rev Clin Oncol. 2022;19:681–97. - PubMed
-
- de Mooij CEM, van der Velden W, de Haan AFJ, Fazel S, van Groningen LFJ, Blijlevens NMA. Grading bloodstream infection risk using citrulline as a biomarker of intestinal mucositis in patients receiving intensive therapy. Bone Marrow Transpl. 2022;57:1373–81. doi: 10.1038/s41409-022-01719-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

