Simultaneous sulfide and methane oxidation by an extremophile
- PMID: 37221165
- PMCID: PMC10205796
- DOI: 10.1038/s41467-023-38699-9
Simultaneous sulfide and methane oxidation by an extremophile
Abstract
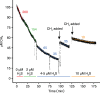
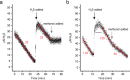
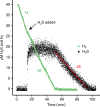
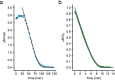
Hydrogen sulfide (H2S) and methane (CH4) are produced in anoxic environments through sulfate reduction and organic matter decomposition. Both gases diffuse upwards into oxic zones where aerobic methanotrophs mitigate CH4 emissions by oxidizing this potent greenhouse gas. Although methanotrophs in myriad environments encounter toxic H2S, it is virtually unknown how they are affected. Here, through extensive chemostat culturing we show that a single microorganism can oxidize CH4 and H2S simultaneously at equally high rates. By oxidizing H2S to elemental sulfur, the thermoacidophilic methanotroph Methylacidiphilum fumariolicum SolV alleviates the inhibitory effects of H2S on methanotrophy. Strain SolV adapts to increasing H2S by expressing a sulfide-insensitive ba3-type terminal oxidase and grows as chemolithoautotroph using H2S as sole energy source. Genomic surveys revealed putative sulfide-oxidizing enzymes in numerous methanotrophs, suggesting that H2S oxidation is much more widespread in methanotrophs than previously assumed, enabling them to connect carbon and sulfur cycles in novel ways.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bagarinao T. Sulfide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquat. Toxicol. 1992;24:21–62. doi: 10.1016/0166-445X(92)90015-F. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

