Acute effects of intravenous DMT in a randomized placebo-controlled study in healthy participants
- PMID: 37221177
- PMCID: PMC10206108
- DOI: 10.1038/s41398-023-02477-4
Acute effects of intravenous DMT in a randomized placebo-controlled study in healthy participants
Abstract
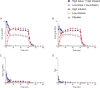
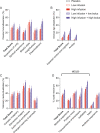
N,N-dimethyltryptamine (DMT) is distinct among classic serotonergic psychedelics because of its short-lasting effects when administered intravenously. Despite growing interest in the experimental and therapeutic use of intravenous DMT, data are lacking on its clinical pharmacology. We conducted a double-blind, randomized, placebo-controlled crossover trial in 27 healthy participants to test different intravenous DMT administration regimens: placebo, low infusion (0.6 mg/min), high infusion (1 mg/min), low bolus + low infusion (15 mg + 0.6 mg/min), and high bolus + high infusion (25 mg + 1 mg/min). Study sessions lasted for 5 h and were separated by at least 1 week. Participant's lifetime use of psychedelics was ≤20 times. Outcome measures included subjective, autonomic, and adverse effects, pharmacokinetics of DMT, and plasma levels of brain-derived neurotropic factor (BDNF) and oxytocin. Low (15 mg) and high (25 mg) DMT bolus doses rapidly induced very intense psychedelic effects that peaked within 2 min. DMT infusions (0.6 or 1 mg/min) without a bolus induced slowly increasing and dose-dependent psychedelic effects that reached plateaus after 30 min. Both bolus doses produced more negative subjective effects and anxiety than infusions. After stopping the infusion, all drug effects rapidly decreased and completely subsided within 15 min, consistent with a short early plasma elimination half-life (t1/2α) of 5.0-5.8 min, followed by longer late elimination (t1/2β = 14-16 min) after 15-20 min. Subjective effects of DMT were stable from 30 to 90 min, despite further increasing plasma concentrations, thus indicating acute tolerance to continuous DMT administration. Intravenous DMT, particularly when administered as an infusion, is a promising tool for the controlled induction of a psychedelic state that can be tailored to the specific needs of patients and therapeutic sessions.Trial registration: ClinicalTrials.gov identifier: NCT04353024.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

