International research to address the challenges of metastatic breast cancer: the AURORA Program (BIG 14-01)
- PMID: 37221256
- PMCID: PMC10205751
- DOI: 10.1038/s41523-023-00548-9
International research to address the challenges of metastatic breast cancer: the AURORA Program (BIG 14-01)
Abstract
Despite recent advances in breast cancer research, we still know little about the mechanisms that lead to metastatic breast cancer (MBC). However, treatment options for patients have increased based on results of recent randomized clinical trials in this setting. Today we have much hope, yet many questions remain unanswered. Conducting a fully academic and international study such as AURORA is very challenging, yet ever more crucial to advancing knowledge about MBC.
Conflict of interest statement
The authors declare competing financial and non-financial interests. C.C., A.I. and T.G. have competing financial interests, as they are employed by Breast International Group (BIG), sponsor of the AURORA program. D.C. has a non-financial competing interest as the Chair of BIG and a competing financial interest since his institution is participating in AURORA with him as the principal investigator. L.N. has a financial competing interest as the Founding Scientific Director of BCRF, the primary funder of the AURORA Program. M.P. has a competing non-financial interest as the Founder and Past Chair of BIG, and one of the principal investigators of AURORA. She has a competing financial interest as the grant recipient for AURORA and her institution is participating in the program.
Figures
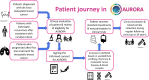

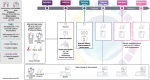
References
Grants and funding
LinkOut - more resources
Full Text Sources

