Novel in vivo endometriotic models associated eutopic endometrium by implanting menstrual blood-derived stromal cells from patients with endometriosis
- PMID: 37221282
- PMCID: PMC10206158
- DOI: 10.1038/s41598-023-35373-4
Novel in vivo endometriotic models associated eutopic endometrium by implanting menstrual blood-derived stromal cells from patients with endometriosis
Erratum in
-
Author Correction: Novel in vivo endometriotic models associated eutopic endometrium by implanting menstrual blood-derived stromal cells from patients with endometriosis.Sci Rep. 2024 Feb 28;14(1):4944. doi: 10.1038/s41598-024-55394-x. Sci Rep. 2024. PMID: 38418526 Free PMC article. No abstract available.
Abstract
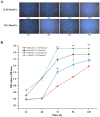
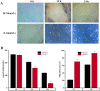
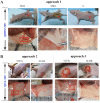
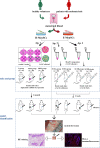
The eutopic endometrium provides novel insights into endometriotic pathophysiology and treatment. However, no in vivo models currently available are suitable for eutopic endometrium in endometriosis. In this study, we present new endometriotic in vivo models associated with eutopic endometrium using menstrual blood-derived stromal cells (MenSCs). First, we isolated endometriotic MenSCs (E-MenSCs) and healthy MenSCs (H-MenSCs) from the menstrual blood of patients with endometriosis (n = 6) and healthy volunteers (n = 6). Then, we identified MenSCs' endometrial stromal cell properties using adipogenic and osteogenic differentiation. A cell counting kit-8 and wound healing assay were used to compare the proliferation and migration capability between E-MenSCs and H-MenSCs. Seventy female nude mice were used to prepare endometriotic models related to eutopic endometrium by implanting E-MenSCs relying on three approaches, including surgical implantation using scaffolds seeded with MenSCs, and subcutaneous injection of MenSCs in the abdomen and the back (n = 10). H-MenSCs or scaffolds only were implanted in control groups (n = 10). One month after the surgical implantation and 1 week after the subcutaneous injection, we evaluated modeling by hematoxylin-eosin (H&E) and immunofluorescent staining of human leukocyte antigen α (HLAA). Fibroblast morphology, lipid droplets, and calcium nodules in E-MenSCs and H-MenSCs identified their endometrial stromal cell properties. We noticed that the proliferation and migration of E-MenSCs were considerably enhanced compared to H-MenSCs (P < 0.05). E-MenSCs implanted in nude mice formed ectopic lesions using three approaches (n = 10; lesions formation rate: 90%, 115%, and 80%; average volumes: 123.60, 27.37, and 29.56 mm3), while H-MenSCs in the nude mice shaped nothing at the implantation sites. Endometrial glands, stroma, and HLAA expression in these lesions further verified the success and applicability of the proposed endometriotic modeling. Findings provide in vitro and in vivo models and paired controls associated with eutopic endometrium in women with endometriosis using E-MenSCs and H-MenSCs. The approach of subcutaneous injection of MenSCs in the abdomen is highlighted due to non-invasive, simple, and safe steps, a short modeling period (1 week), and an excellent modeling success rate (115%), which could improve the repeats and success of endometriotic nude mice model and shorten the modeling period. These novel models could nearly intimate human eutopic endometrial mesenchymal stromal cells in the progress of endometriosis, opening a new path for disease pathology and treatment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
T-cadherin inhibits invasion and migration of endometrial stromal cells in endometriosis.Hum Reprod. 2020 Jan 1;35(1):145-156. doi: 10.1093/humrep/dez252. Hum Reprod. 2020. PMID: 31886853
-
Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis-like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis.Hum Reprod. 2019 Jan 1;34(1):56-68. doi: 10.1093/humrep/dey336. Hum Reprod. 2019. PMID: 30496412 Free PMC article.
-
Reduced α-2,6 sialylation regulates cell migration in endometriosis.Hum Reprod. 2019 Mar 1;34(3):479-490. doi: 10.1093/humrep/dey391. Hum Reprod. 2019. PMID: 30753458
-
Cancer-associated mutations in endometriosis: shedding light on the pathogenesis and pathophysiology.Hum Reprod Update. 2020 Apr 15;26(3):423-449. doi: 10.1093/humupd/dmz047. Hum Reprod Update. 2020. PMID: 32154564 Review.
-
The Different Gene Expression Profile in the Eutopic and Ectopic Endometrium Sheds New Light on the Endometrial Seed in Endometriosis.Biomedicines. 2024 Jun 8;12(6):1276. doi: 10.3390/biomedicines12061276. Biomedicines. 2024. PMID: 38927483 Free PMC article. Review.
Cited by
-
Intra-ovarian injection of autologous menstrual blood-derived-mesenchymal stromal cells: a safe and promising method to improve pregnancy rate in poor ovarian responders.Stem Cell Res Ther. 2025 Apr 12;16(1):171. doi: 10.1186/s13287-025-04278-6. Stem Cell Res Ther. 2025. PMID: 40221741 Free PMC article.
-
Unveiling the fibrotic puzzle of endometriosis: An overlooked concern calling for prompt action.F1000Res. 2024 Dec 3;13:721. doi: 10.12688/f1000research.152368.3. eCollection 2024. F1000Res. 2024. PMID: 39669683 Free PMC article. Review.
-
Modelling Endometriosis Using In Vitro and In Vivo Systems.Int J Mol Sci. 2025 Jan 11;26(2):580. doi: 10.3390/ijms26020580. Int J Mol Sci. 2025. PMID: 39859296 Free PMC article. Review.
References
-
- Chinese Obstetricians and Gynecologists Association; Cooperative Group of Endometriosis, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association Guidelines for the diagnosis and treatment of endometriosis (third edition) Chin. J. Obstet. Gynecol. 2021;56:812–824.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

