Involvement of cortical input to the rostromedial tegmental nucleus in aversion to foot shock
- PMID: 37221326
- PMCID: PMC10425416
- DOI: 10.1038/s41386-023-01612-5
Involvement of cortical input to the rostromedial tegmental nucleus in aversion to foot shock
Abstract
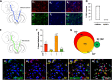
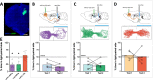
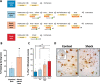
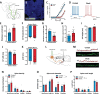
The rostromedial tegmental nucleus (RMTg) encodes negative reward prediction error (RPE) and plays an important role in guiding behavioral responding to aversive stimuli. Previous research has focused on regulation of RMTg activity by the lateral habenula despite studies revealing RMTg afferents from other regions including the frontal cortex. The current study provides a detailed anatomical and functional analysis of cortical input to the RMTg of male rats. Retrograde tracing uncovered dense cortical input to the RMTg spanning the medial prefrontal cortex, the orbitofrontal cortex and anterior insular cortex. Afferents were most dense in the dorsomedial subregion of the PFC (dmPFC), an area that is also implicated in both RPE signaling and aversive responding. RMTg-projecting dmPFC neurons originate in layer V, are glutamatergic, and collateralize to select brain regions. In-situ mRNA hybridization revealed that neurons in this circuit are predominantly D1 receptor-expressing with a high degree of D2 receptor colocalization. Consistent with cFos induction in this neural circuit during exposure to foot shock and shock-predictive cues, optogenetic stimulation of dmPFC terminals in the RMTg drove avoidance. Lastly, acute slice electrophysiology and morphological studies revealed that exposure to repeated foot shock resulted in significant physiological and structural changes consistent with a loss of top-down modulation of RMTg-mediated signaling. Altogether, these data reveal the presence of a prominent cortico-subcortical projection involved in adaptive behavioral responding to aversive stimuli such as foot shock and provide a foundation for future work aimed at exploring alterations in circuit function in diseases characterized by deficits in cognitive control over reward and aversion.
© 2023. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Role for the Rostromedial Tegmental Nucleus in Signaling the Aversive Properties of Alcohol.Alcohol Clin Exp Res. 2016 Aug;40(8):1651-61. doi: 10.1111/acer.13140. Epub 2016 Jul 8. Alcohol Clin Exp Res. 2016. PMID: 27388762 Free PMC article.
-
Learning From One's Mistakes: A Dual Role for the Rostromedial Tegmental Nucleus in the Encoding and Expression of Punished Reward Seeking.Biol Psychiatry. 2017 Jun 15;81(12):1041-1049. doi: 10.1016/j.biopsych.2016.10.018. Epub 2016 Oct 21. Biol Psychiatry. 2017. PMID: 27931744 Free PMC article.
-
Alcohol withdrawal drives depressive behaviors by activating neurons in the rostromedial tegmental nucleus.Neuropsychopharmacology. 2019 Jul;44(8):1464-1475. doi: 10.1038/s41386-019-0378-8. Epub 2019 Mar 31. Neuropsychopharmacology. 2019. PMID: 30928995 Free PMC article.
-
The Rostromedial Tegmental Nucleus: Anatomical Studies and Roles in Sleep and Substance Addictions in Rats and Mice.Nat Sci Sleep. 2020 Dec 24;12:1215-1223. doi: 10.2147/NSS.S278026. eCollection 2020. Nat Sci Sleep. 2020. PMID: 33380853 Free PMC article. Review.
-
The rostromedial tegmental (RMTg) "brake" on dopamine and behavior: A decade of progress but also much unfinished work.Neuropharmacology. 2021 Oct 15;198:108763. doi: 10.1016/j.neuropharm.2021.108763. Epub 2021 Aug 22. Neuropharmacology. 2021. PMID: 34433088 Free PMC article. Review.
Cited by
-
Activation of the tail of the ventral tegmental area in response to pup predicting cues in maternal rats.Brain Struct Funct. 2025 Jul 24;230(7):121. doi: 10.1007/s00429-025-02987-5. Brain Struct Funct. 2025. PMID: 40705119 Free PMC article.
-
The imprint of dissociative seizures on the brain.Neuroimage Clin. 2024;43:103664. doi: 10.1016/j.nicl.2024.103664. Epub 2024 Aug 29. Neuroimage Clin. 2024. PMID: 39226702 Free PMC article.
-
The prelimbic prefrontal cortex mediates the development of lasting social avoidance as a consequence of social threat conditioning.Neuropsychopharmacology. 2025 Feb 27. doi: 10.1038/s41386-025-02073-8. Online ahead of print. Neuropsychopharmacology. 2025. PMID: 40016364
-
Chronic ethanol exposure produces long-lasting, subregion-specific physiological adaptations in RMTg-projecting mPFC neurons.Neuropharmacology. 2024 Nov 15;259:110098. doi: 10.1016/j.neuropharm.2024.110098. Epub 2024 Aug 6. Neuropharmacology. 2024. PMID: 39117106 Free PMC article.
References
-
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–96. doi: 10.1002/cne.21891. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

