Circulating anti-hypothalamus antibodies in celiac patients: tissue transglutaminase friend or foe?
- PMID: 37221348
- PMCID: PMC10667380
- DOI: 10.1007/s12026-023-09394-0
Circulating anti-hypothalamus antibodies in celiac patients: tissue transglutaminase friend or foe?
Abstract
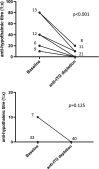
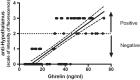
Celiac disease (CD) is an autoimmune disease with inflammatory characteristics, having a condition of chronic malabsorption, affecting approximately 1% of the population at any age. In recent years, a concrete correlation between eating disorders and CD has emerged. Hypothalamus plays a central role in determining eating behaviour, regulating appetite and, consequently, food intake. One hundred and ten sera from celiac patients (40 active and 70 following a gluten-free diet) were tested for the presence of autoantibodies against primate hypothalamic periventricular neurons by immunofluorescence and by a home-made ELISA assay. In addition, ghrelin was measured by ELISA. As control, 45 blood serums from healthy age matched were analysed. Among active CD, all patients resulted positive for anti-hypothalamus autoantibodies and sera showed significantly higher levels of ghrelin. All of the free-gluten CD were negative for anti-hypothalamus autoantibodies and had low levels of ghrelin, as well as healthy controls. Of interest, anti-hypothalamic autoantibodies directly correlate with anti-tTG amounts and with mucosal damage. In addition, competition assays with recombinant tTG showed a drastically reduction of anti-hypothalamic serum reactivity. Finally, ghrelin levels are increased in CD patients and correlated with anti-tTG autoantibodies and anti-hypothalamus autoantibodies. This study demonstrates for the first time the presence of anti-hypothalamus antibodies and their correlation with the severity of the CD. It also allows us to hypothesize the role of tTG as a putative autoantigen expressed by hypothalamic neurons.
Keywords: Active CD; Free-gluten CD; Anti-hypothalamus autoantibodies; Anti-tTG autoantibodies; Mucosal damage; Ghrelin.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Antibodies against neo-epitope of microbial and human transglutaminase complexes as biomarkers of childhood celiac disease.Clin Exp Immunol. 2020 Mar;199(3):294-302. doi: 10.1111/cei.13394. Epub 2019 Nov 11. Clin Exp Immunol. 2020. PMID: 31663117 Free PMC article.
-
Antitissue transglutaminase and antithyroid autoantibodies in children with Down syndrome and celiac disease.J Pediatr Gastroenterol Nutr. 2005 Feb;40(2):170-4; discussion 125-7. doi: 10.1097/00005176-200502000-00016. J Pediatr Gastroenterol Nutr. 2005. PMID: 15699691
-
Serum IL-21 levels from celiac disease patients correlates with anti-tTG IgA autoantibodies and mucosal damage.Autoimmunity. 2020 Jun;53(4):225-230. doi: 10.1080/08916934.2020.1736047. Epub 2020 Mar 11. Autoimmunity. 2020. PMID: 32157915
-
Tissue transglutaminase antibodies in celiac disease: focus on the pediatric population.Drugs Today (Barc). 2011 Sep;47(9):683-91. doi: 10.1358/dot.2011.47.9.1682709. Drugs Today (Barc). 2011. PMID: 21971542 Review.
-
Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients With Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: a Meta-analysis.Gastroenterology. 2017 Sep;153(3):689-701.e1. doi: 10.1053/j.gastro.2017.05.015. Epub 2017 May 22. Gastroenterology. 2017. PMID: 28545781 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

