Synergistic action of phages and lytic proteins with antibiotics: a combination strategy to target bacteria and biofilms
- PMID: 37221517
- PMCID: PMC10204329
- DOI: 10.1186/s12866-023-02881-2
Synergistic action of phages and lytic proteins with antibiotics: a combination strategy to target bacteria and biofilms
Abstract
Background: Multidrug-resistant bacteria continue to emerge owing to the abuse of antibiotics and have a considerable negative impact on people and the environment. Bacteria can easily form biofilms to improve their survival, which reduces the efficacy of antibacterial drugs. Proteins such as endolysins and holins have been shown to have good antibacterial activity and effectively removal bacterial biofilms and reduce the production of drug-resistant bacteria. Recently, phages and their encoded lytic proteins have attracted attention as potential alternative antimicrobial agents. The aim of the present study was to investigate the sterilising efficacy of phages (SSE1, SGF2, and SGF3) and their encoded lytic proteins (lysozyme and holin), and to further explore their potential in combination with antibiotics. To the ultimate aim is to reduce or replace the use of antibiotics and provide more materials and options for sterilisation.
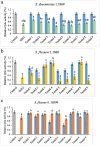
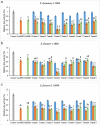
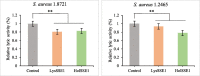
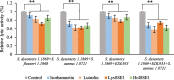
Results: Phages and their encoded lytic proteins were confirmed to have great advantages in sterilisation, and all exhibited significant potential for reducing bacterial resistance. Previous studies on the host spectrum demonstrated the bactericidal efficacy of three Shigella phages (SSE1, SGF2, and SGF3) and two lytic proteins (LysSSE1 and HolSSE1). In this study, we investigated the bactericidal effects on planktonic bacteria and bacterial biofilms. A combined sterilisation application of antibiotics, phages, and lytic proteins was performed. The results showed that phages and lytic proteins had better sterilisation effects than antibiotics with 1/2 minimum inhibitory concentrations (MIC) and their effect was further improved when used together with antibiotics. The best synergy was shown when combined with β- lactam antibiotics, which might be related to their mechanism of sterilising action. This approach ensures a bactericidal effect at low antibiotic concentrations.
Conclusions: This study strengthens the idea that phages and lytic proteins can significantly sterilise bacteria in vitro and achieve synergistic sterilisation effects with specific antibiotics. Therefore, a suitable combination strategy may decrease the risk of drug resistance.
Keywords: Antibiotic; Phages and lytic proteins; Synergistic sterilization.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Yu X, Zhao J, Fan D. A dissolving microneedle patch for Antibiotic/Enzymolysis/Photothermal triple therapy against bacteria and their biofilms. Chem Eng J. 2022;437:135475. doi: 10.1016/j.cej.2022.135475. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

