Menopause causes metabolic and cognitive impairments in a chronic cerebral hypoperfusion model of vascular contributions to cognitive impairment and dementia
- PMID: 37221553
- PMCID: PMC10204285
- DOI: 10.1186/s13293-023-00518-7
Menopause causes metabolic and cognitive impairments in a chronic cerebral hypoperfusion model of vascular contributions to cognitive impairment and dementia
Abstract
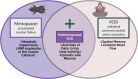
Background: The vast majority of women with dementia are post-menopausal. Despite clinical relevance, menopause is underrepresented in rodent models of dementia. Before menopause, women are less likely than men to experience strokes, obesity, and diabetes-known risk factors for vascular contributions to cognitive impairment and dementia (VCID). During menopause, ovarian estrogen production stops and the risk of developing these dementia risk factors spikes. Here, we aimed to determine if menopause worsens cognitive impairment in VCID. We hypothesized that menopause would cause metabolic dysfunction and increase cognitive impairment in a mouse model of VCID.
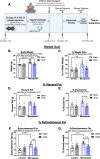
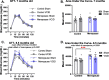
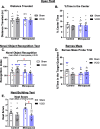
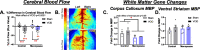
Methods: We performed a unilateral common carotid artery occlusion surgery to produce chronic cerebral hypoperfusion and model VCID in mice. We used 4-vinylcyclohexene diepoxide to induce accelerated ovarian failure and model menopause. We evaluated cognitive impairment using behavioral tests including novel object recognition, Barnes maze, and nest building. To assess metabolic changes, we measured weight, adiposity, and glucose tolerance. We explored multiple aspects of brain pathology including cerebral hypoperfusion and white matter changes (commonly observed in VCID) as well as changes to estrogen receptor expression (which may mediate altered sensitivity to VCID pathology post-menopause).
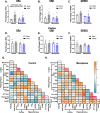
Results: Menopause increased weight gain, glucose intolerance, and visceral adiposity. VCID caused deficits in spatial memory regardless of menopausal status. Post-menopausal VCID specifically led to additional deficits in episodic-like memory and activities of daily living. Menopause did not alter resting cerebral blood flow on the cortical surface (assessed by laser speckle contrast imaging). In the white matter, menopause decreased myelin basic protein gene expression in the corpus callosum but did not lead to overt white matter damage (assessed by Luxol fast blue). Menopause did not significantly alter estrogen receptor expression (ERα, ERβ, or GPER1) in the cortex or hippocampus.
Conclusions: Overall, we have found that the accelerated ovarian failure model of menopause caused metabolic impairment and cognitive deficits in a mouse model of VCID. Further studies are needed to identify the underlying mechanism. Importantly, the post-menopausal brain still expressed estrogen receptors at normal (pre-menopausal) levels. This is encouraging for any future studies attempting to reverse the effects of estrogen loss by activating brain estrogen receptors.
Keywords: Cognitive impairment; Dementia; Estrogen; Hypoperfusion; Menopause; Metabolic; Myelin; Neuroinflammation; Neuroscience; Vascular.
Plain language summary
Nearly all women with dementia are menopausal. Reduced blood flow to the brain, resulting from damaged blood vessels, can lead to vascular dementia. Vascular dementia is the second most common cause of dementia. Before menopause, women are less likely than men to experience strokes, obesity, and diabetes—known risk factors for vascular dementia. During menopause, estrogen levels drop and the risk of developing these dementia risk factors increases. The goal of this study was to determine how menopause impacts risk factors (obesity, diabetes), memory and brain pathology in vascular dementia. This study used mouse models of vascular dementia and menopause. Menopause increased weight gain and other indicators of poor metabolic health. In mice with vascular dementia, menopausal mice had worse memory than pre-menopausal mice. After menopause, the brain still expressed estrogen receptors at normal (pre-menopausal) levels. This is encouraging for any future studies attempting to reverse the effects of estrogen loss by activating brain estrogen receptors.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Chatterjee S, Peters SA, Woodward M, Mejia Arango S, Batty GD, Beckett N, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307. doi: 10.2337/dc15-1588. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

