Microglia degrade Tau oligomers deposit via purinergic P2Y12-associated podosome and filopodia formation and induce chemotaxis
- PMID: 37221563
- PMCID: PMC10204346
- DOI: 10.1186/s13578-023-01028-0
Microglia degrade Tau oligomers deposit via purinergic P2Y12-associated podosome and filopodia formation and induce chemotaxis
Abstract
Background: Tau protein forms neurofibrillary tangles and becomes deposited in the brain during Alzheimer's disease (AD). Tau oligomers are the most reactive species, mediating neurotoxic and inflammatory activity. Microglia are the immune cells in the central nervous system, sense the extracellular Tau via various cell surface receptors. Purinergic P2Y12 receptor can directly interact with Tau oligomers and mediates microglial chemotaxis via actin remodeling. The disease-associated microglia are associated with impaired migration and express a reduced level of P2Y12, but elevate the level of reactive oxygen species and pro-inflammatory cytokines.
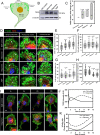
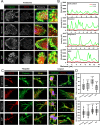
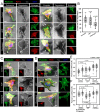
Results: Here, we studied the formation and organization of various actin microstructures such as-podosome, filopodia and uropod in colocalization with actin nucleator protein Arp2 and scaffold protein TKS5 in Tau-induced microglia by fluorescence microscopy. Further, the relevance of P2Y12 signaling either by activation or blockage was studied in terms of actin structure formations and Tau deposits degradation by N9 microglia. Extracellular Tau oligomers facilitate the microglial migration via Arp2-associated podosome and filopodia formation through the involvement of P2Y12 signaling. Similarly, Tau oligomers induce the TKS5-associated podosome clustering in microglial lamella in a time-dependent manner. Moreover, the P2Y12 was evidenced to localize with F-actin-rich podosome and filopodia during Tau-deposit degradation. The blockage of P2Y12 signaling resulted in decreased microglial migration and Tau-deposit degradation.
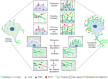
Conclusions: The P2Y12 signaling mediate the formation of migratory actin structures like- podosome and filopodia to exhibit chemotaxis and degrade Tau deposit. These beneficial roles of P2Y12 in microglial chemotaxis, actin network remodeling and Tau clearance can be intervened as a therapeutic target in AD.
Keywords: Filopodia; Microglia; Migration; P2Y12; Podosome; Tau oligomers.
© 2023. The Author(s).
Conflict of interest statement
The authors declared that there is no competing interests associated with this study.
Figures



Similar articles
-
Tau aggregates improve the Purinergic receptor P2Y12-associated podosome rearrangements in microglial cells.Biochim Biophys Acta Mol Cell Res. 2023 Jun;1870(5):119477. doi: 10.1016/j.bbamcr.2023.119477. Epub 2023 Apr 13. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 37061007
-
Microglial remodeling of actin network by Tau oligomers, via G protein-coupled purinergic receptor, P2Y12R-driven chemotaxis.Traffic. 2021 May;22(5):153-170. doi: 10.1111/tra.12784. Epub 2021 Feb 18. Traffic. 2021. PMID: 33527700
-
G-protein coupled purinergic P2Y12 receptor interacts and internalizes TauRD-mediated by membrane-associated actin cytoskeleton remodeling in microglia.Eur J Cell Biol. 2022 Apr;101(2):151201. doi: 10.1016/j.ejcb.2022.151201. Epub 2022 Jan 25. Eur J Cell Biol. 2022. PMID: 35101770 Review.
-
Actin-mediated Microglial Chemotaxis via G-Protein Coupled Purinergic Receptor in Alzheimer's Disease.Neuroscience. 2020 Nov 10;448:325-336. doi: 10.1016/j.neuroscience.2020.09.024. Epub 2020 Sep 14. Neuroscience. 2020. PMID: 32941933 Review.
-
Phagocytosis of full-length Tau oligomers by Actin-remodeling of activated microglia.J Neuroinflammation. 2020 Jan 8;17(1):10. doi: 10.1186/s12974-019-1694-y. J Neuroinflammation. 2020. PMID: 31915009 Free PMC article.
Cited by
-
Potential targets of microglia in the treatment of neurodegenerative diseases: Mechanism and therapeutic implications.Neural Regen Res. 2026 Apr 1;21(4):1497-1511. doi: 10.4103/NRR.NRR-D-24-01343. Epub 2025 Mar 25. Neural Regen Res. 2026. PMID: 40145977 Free PMC article.
-
Microglia internalize tau monomers and fibrils using distinct receptors but similar mechanisms.Alzheimers Dement. 2025 Feb;21(2):e14418. doi: 10.1002/alz.14418. Epub 2024 Dec 23. Alzheimers Dement. 2025. PMID: 39713861 Free PMC article.
-
Microglial purinergic signaling in Alzheimer's disease.Purinergic Signal. 2024 Jun 24. doi: 10.1007/s11302-024-10029-8. Online ahead of print. Purinergic Signal. 2024. PMID: 38910192 Review.
-
Glial phagocytosis for synapse and toxic proteins in neurodegenerative diseases.Mol Neurodegener. 2025 Jul 9;20(1):81. doi: 10.1186/s13024-025-00870-9. Mol Neurodegener. 2025. PMID: 40629407 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

