Extracellular vesicles: emerging roles, biomarkers and therapeutic strategies in fibrotic diseases
- PMID: 37221595
- PMCID: PMC10205568
- DOI: 10.1186/s12951-023-01921-3
Extracellular vesicles: emerging roles, biomarkers and therapeutic strategies in fibrotic diseases
Abstract
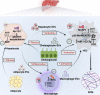
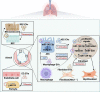
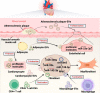
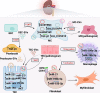
Extracellular vesicles (EVs), a cluster of cell-secreted lipid bilayer nanoscale particles, universally exist in body fluids, as well as cell and tissue culture supernatants. Over the past years, increasing attention have been paid to the important role of EVs as effective intercellular communicators in fibrotic diseases. Notably, EV cargos, including proteins, lipids, nucleic acids, and metabolites, are reported to be disease-specific and can even contribute to fibrosis pathology. Thus, EVs are considered as effective biomarkers for disease diagnosis and prognosis. Emerging evidence shows that EVs derived from stem/progenitor cells have great prospects for cell-free therapy in various preclinical models of fibrotic diseases and engineered EVs can improve the targeting and effectiveness of their treatment. In this review, we will focus on the biological functions and mechanisms of EVs in the fibrotic diseases, as well as their potential as novel biomarkers and therapeutic strategies.
Keywords: Diagnosis; Extracellular vesicles; Fibrosis; Pathogenesis; Therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Extracellular Vesicles in Cardiovascular Theranostics.Theranostics. 2017 Sep 26;7(17):4168-4182. doi: 10.7150/thno.21274. eCollection 2017. Theranostics. 2017. PMID: 29158817 Free PMC article. Review.
-
Extracellular Vesicles in Idiopathic Pulmonary Fibrosis: Pathogenesis, Biomarkers and Innovative Therapeutic Strategies.Int J Nanomedicine. 2024 Nov 25;19:12593-12614. doi: 10.2147/IJN.S491335. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39619058 Free PMC article. Review.
-
Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification.J Nanobiotechnology. 2023 Sep 16;21(1):334. doi: 10.1186/s12951-023-02081-0. J Nanobiotechnology. 2023. PMID: 37717008 Free PMC article. Review.
-
Recent advances in the roles of extracellular vesicles in cardiovascular diseases: pathophysiological mechanisms, biomarkers, and cell-free therapeutic strategy.Mol Med. 2025 May 5;31(1):169. doi: 10.1186/s10020-025-01200-x. Mol Med. 2025. PMID: 40325357 Free PMC article. Review.
-
Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases.Biomolecules. 2021 Mar 5;11(3):388. doi: 10.3390/biom11030388. Biomolecules. 2021. PMID: 33808038 Free PMC article. Review.
Cited by
-
Methods for Extracellular Vesicle Isolation: Relevance for Encapsulated miRNAs in Disease Diagnosis and Treatment.Genes (Basel). 2025 Mar 12;16(3):330. doi: 10.3390/genes16030330. Genes (Basel). 2025. PMID: 40149481 Free PMC article. Review.
-
Extracellular vesicles: opening up a new perspective for the diagnosis and treatment of mitochondrial dysfunction.J Nanobiotechnology. 2024 Aug 14;22(1):487. doi: 10.1186/s12951-024-02750-8. J Nanobiotechnology. 2024. PMID: 39143493 Free PMC article. Review.
-
Rigorous Process for Isolation of Gut-Derived Extracellular Vesicles (EVs) and the Effect on Latent HIV.Cells. 2025 Apr 9;14(8):568. doi: 10.3390/cells14080568. Cells. 2025. PMID: 40277894 Free PMC article.
-
Noninvasive in vivo imaging of macrophages: understanding tumor microenvironments and delivery of therapeutics.Biomark Res. 2025 Jan 26;13(1):20. doi: 10.1186/s40364-025-00735-9. Biomark Res. 2025. PMID: 39865337 Free PMC article. Review.
-
Extracellular Vesicle Preparation and Analysis: A State-of-the-Art Review.Adv Sci (Weinh). 2024 Aug;11(30):e2401069. doi: 10.1002/advs.202401069. Epub 2024 Jun 14. Adv Sci (Weinh). 2024. PMID: 38874129 Free PMC article. Review.
References
-
- Van der Heyden A, Chanthavong P, Angles-Cano E, Bonnet H, Dejeu J, Cras A, et al. Grafted dinuclear zinc complexes for selective recognition of phosphatidylserine: application to the capture of extracellular membrane microvesicles. J Inorg Biochem. 2023;239:112065. doi: 10.1016/j.jinorgbio.2022.112065. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 202210299082Z/Innovation Training Programme for University Students
- 82272179/National Natural Science Foundation of China
- SS2018003/Priority Academic Program Development of Jiangsu Higher Education Institutions (Phase III) and Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application
LinkOut - more resources
Full Text Sources
Miscellaneous

