Acetate and propionate effects in response to LPS in a porcine intestinal co-culture model
- PMID: 37221609
- PMCID: PMC10207778
- DOI: 10.1186/s40813-023-00316-y
Acetate and propionate effects in response to LPS in a porcine intestinal co-culture model
Abstract
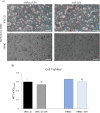
Background: The interest in acetate and propionate as short chain fatty acids (SCFA) derives from research on alternative strategies to the utilization of antibiotics in pig farms. SCFA have a protective role on the intestinal epithelial barrier and improve intestinal immunity by regulating the inflammatory and immune response. This regulation is associated with an increase in intestinal barrier integrity, mediated by the enhancement of tight junction protein (TJp) functions, which prevent the passage of pathogens through the paracellular space. The purpose of this study was to evaluate the effect of in vitro supplementation with SCFA (5 mM acetate and 1 mM propionate) on viability, nitric oxide (NO) release (oxidative stress), NF-κB gene expression, and gene and protein expression of major TJp (occludin [OCLN], zonula occludens-1 [ZO-1], and claudin-4 [CLDN4]) in a porcine intestinal epithelial cell (IPEC-J2) and peripheral blood mononuclear cell (PBMC) co-culture model upon LPS stimulation, through which an acute inflammatory state was simulated.
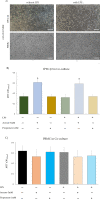
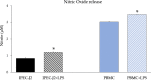
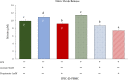
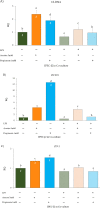
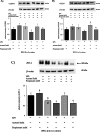
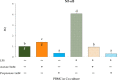
Results: Firstly, the inflammatory stimulus induced by LPS evaluated in the IPEC-J2 monoculture was characterized by a reduction of viability, gene expression of TJp and OCLN protein synthesis, and an increase of NO release. The response evaluated in the co-culture showed that acetate positively stimulated the viability of both untreated and LPS-stimulated IPEC-J2 and reduced the release of NO in LPS-stimulated cells. Acetate also promoted an increase of gene expression of CLDN4, ZO-1, and OCLN, and protein synthesis of CLDN4, OCLN and ZO-1 in untreated and LPS-stimulated cells. Propionate induced a reduction of NO release in both untreated and LPS-stimulated IPEC-J2. In untreated cells, propionate induced an increase of TJp gene expression and of CLDN4 and OCLN protein synthesis. Contrarily, propionate in LPS-stimulated cells induced an increase of CLDN4 and OCLN gene expression and protein synthesis. PBMC were influenced by acetate and propionate supplementation, in that NF-κB expression was strongly downregulated in LPS-stimulated cells.
Conclusions: The present study demonstrates the protective effect of acetate and propionate upon acute inflammation by regulating epithelial tight junction expression and protein synthesis in a co-culture model, which simulates the in vivo interaction between epithelial intestinal cells and local immune cells.
Keywords: Co-culture system; IPEC-J2; Intestinal epithelial barrier function; PBMC; SCFA; Tight junctions.
© 2023. The Author(s).
Conflict of interest statement
The authors declare there are no competing interests.
Figures


References
-
- Zhang G, Zhao JB, Dong WX, Song XM, Lin G, Li DF, et al. Yeast-derived mannan-rich fraction as an alternative for zinc oxide to alleviate diarrhea incidence and improve growth performance in weaned pigs. Anim Feed Sci Technol. 2021 doi: 10.1016/j.anifeedsci.2021.115111. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

