A different pattern of clinical, muscle pathology and brain MRI findings in MELAS with mt-ND variants
- PMID: 37221696
- PMCID: PMC10270267
- DOI: 10.1002/acn3.51787
A different pattern of clinical, muscle pathology and brain MRI findings in MELAS with mt-ND variants
Abstract
Objective: To explore the clinical characteristics of mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) caused by mitochondrial DNA-encoded complex I subunit (mt-ND) variants.
Methods: In this retrospective study, the clinical, myopathological and brain MRI features of patients with MELAS caused by mt-ND variants (MELAS-mtND) were collected and compared with those of MELAS patients carrying the m.3243A > G variant (MELAS-A3243G).
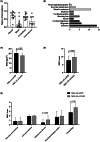
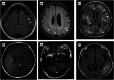
Result: A total of 18 MELAS-mtND patients (female: 7; median age: 24.5 years) represented 15.9% (n = 113) of all patients with MELAS caused by mtDNA variants in our neuromuscular center from January 2012 to June 2022. In this MELAS-mtND cohort, the two most common variants were m.10191 T > C (4/18, 22.2%) and m.13513 G > A (3/18, 16.7%). The most frequent symptoms were seizures (14/18, 77.8%) and muscle weakness (11/18, 61.1%). Compared with 87 MELAS-A3243G patients, MELAS-mtND patients were significantly more likely to have a variant that was absent in blood cells (40% vs. 1.4%). Furthermore, MELAS-mtND patients had a significantly lower MDC score (7.8 ± 2.7 vs. 9.8 ± 1.9); less hearing loss (27.8% vs. 54.0%), diabetes (11.1% vs. 37.9%), and migraine (33.3% vs. 62.1%); less short stature (males ≤ 165 cm; females ≤ 155 cm; 23.1% vs. 60.8%) and higher body mass index (20.4 ± 2.5 vs. 17.8 ± 2.7). MELAS-mtND patients had significantly more normal muscle pathology (31.3% vs. 4.1%) and fewer RRFs/RBFs (62.5% vs. 91.9%), COX-deficient fibers/blue fibers (25.0% vs. 85.1%) and SSVs (50.0% vs. 81.1%). Moreover, brain MRI evaluated at the first stroke-like episode showed significantly more small cortical lesions in MELAS-mtND patients (66.7% vs. 12.2%).
Interpretation: Our results suggested that MELAS-mtND patients have distinct clinical, myopathological and brain MRI features compared with MELAS-A3243G patients.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
All authors report no competing interests.
Figures


References
-
- La Morgia C, Maresca A, Caporali L, Valentino ML, Carelli V. Mitochondrial diseases in adults. J Intern Med. 2020;287(6):592‐608. - PubMed
-
- Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348(6302):651‐653. - PubMed
-
- El‐Hattab AW, Adesina AM, Jones J, Scaglia F. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab. 2015. Sep‐Oct;116(1–2):4‐12. - PubMed
-
- Santorelli FM, Tanji K, Kulikova R, et al. Identification of a novel mutation in the mtDNA ND5 gene associated with MELAS. Biochem Biophys Res Commun. 1997;238(2):326‐328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

