Preferences and acceptability for long-acting PrEP agents among pregnant and postpartum women with experience using daily oral PrEP in South Africa and Kenya
- PMID: 37221983
- PMCID: PMC10206415
- DOI: 10.1002/jia2.26088
Preferences and acceptability for long-acting PrEP agents among pregnant and postpartum women with experience using daily oral PrEP in South Africa and Kenya
Abstract
Introduction: Long-acting pre-exposure prophylaxis (PrEP) options could overcome barriers to oral PrEP persistence during pregnancy and postpartum. We evaluated long-acting PrEP preferences among oral PrEP-experienced pregnant and postpartum women in South Africa and Kenya, countries with high PrEP coverage with pending regulatory approvals for long-acting injectable cabotegravir and the dapivirine vaginal ring (approved in South Africa, under review in Kenya).
Methods: From September 2021 to February 2022, we surveyed pregnant and postpartum women enrolled in oral PrEP studies in South Africa and Kenya. We evaluated oral PrEP attitudes and preferences for long-acting PrEP methods in multivariable logistic regression models adjusting for maternal age and country.
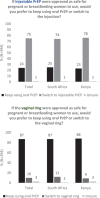
Results: We surveyed 190 women in South Africa (67% postpartum; median age 27 years [IQR = 22-32]) and 204 women in Kenya (79% postpartum; median age 29 years [IQR = 25-33]). Seventy-five percent of participants reported oral PrEP use within the last 30 days. Overall, forty-nine percent of participants reported negative oral PrEP attributes, including side effects (21% South Africa, 30% Kenya) and pill burden (20% South Africa, 25% Kenya). Preferred PrEP attributes included long-acting method, effectiveness, safety while pregnant and breastfeeding, and free medication. Most participants (75%, South Africa and Kenya) preferred a potential long-acting injectable over oral PrEP, most frequently for a longer duration of effectiveness in South Africa (87% South Africa, 42% Kenya) versus discretion in Kenya (5% South Africa, 49% Kenya). Eighty-seven percent of participants preferred oral PrEP over a potential long-acting vaginal ring, mostly due to concern about possible discomfort with vaginal insertion (82% South Africa, 48% Kenya). Significant predictors of long-acting PrEP preference included past use of injectable contraceptive (aOR = 2.48, 95% CI: 1.34, 4.57), disliking at least one oral PrEP attribute (aOR = 1.72, 95% CI: 1.05, 2.80) and preferring infrequent PrEP use (aOR = 1.58, 95% CI: 0.94, 2.65).
Conclusions: Oral PrEP-experienced pregnant and postpartum women expressed a theoretical preference for long-acting injectable PrEP over other modalities, demonstrating potential acceptability among a key population who must be at the forefront of injectable PrEP rollout. Reasons for PrEP preferences differed by country, emphasizing the importance of increasing context-specific options and choice of PrEP modalities for pregnant and postpartum women.
Keywords: Kenya; PrEP; South Africa; breastfeeding; long-acting; pregnancy.
© 2023 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Conflict of interest statement
The PrEP‐PP study received the study drug (Truvada®) from Gilead Sciences (Foster City, CA, USA).
Figures
References
-
- UNAIDS. (n.d.) . Start Free Stay Free AIDS Free ‐ 2020 Progress Report. Retrieved May 10, 2022. https://www.unaids.org/sites/default/files/media_asset/start‐free‐stay‐f...
-
- Woldesenbet S, Kufa‐Chakezha T, Lombard C, Manda S, Cheyip M, Ayalew K, et al. Recent HIV infection among pregnant women in the 2017 antenatal sentinel crosssectional survey, South Africa: assay‐based incidence measurement. PLoS One. 2021;. 16(4):e0249953. 10.1371/journal.pone.0249953 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

