Increasing brain N-acetylneuraminic acid alleviates hydrocephalus-induced neurological deficits
- PMID: 37222223
- PMCID: PMC10580356
- DOI: 10.1111/cns.14253
Increasing brain N-acetylneuraminic acid alleviates hydrocephalus-induced neurological deficits
Abstract
Aims: This metabolomic study aimed to evaluate the role of N-acetylneuraminic acid (Neu5Ac) in the neurological deficits of normal pressure hydrocephalus (NPH) and its potential therapeutic effect.
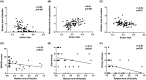
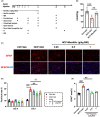
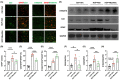
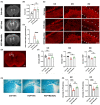
Methods: We analyzed the metabolic profiles of NPH using cerebrospinal fluid with multivariate and univariate statistical analyses in a set of 42 NPH patients and 38 controls. We further correlated the levels of differential metabolites with severity-related clinical parameters, including the normal pressure hydrocephalus grading scale (NPHGS). We then established kaolin-induced hydrocephalus in mice and treated them using N-acetylmannosamine (ManNAc), a precursor of Neu5Ac. We examined brain Neu5Ac, astrocyte polarization, demyelination, and neurobehavioral outcomes to explore its therapeutic effect.
Results: Three metabolites were significantly altered in NPH patients. Only decreased Neu5Ac levels were correlated with NPHGS scores. Decreased brain Neu5Ac levels have been observed in hydrocephalic mice. Increasing brain Neu5Ac by ManNAc suppressed the activation of astrocytes and promoted their transition from A1 to A2 polarization. ManNAc also attenuated the periventricular white matter demyelination and improved neurobehavioral outcomes in hydrocephalic mice.
Conclusion: Increasing brain Neu5Ac improved the neurological outcomes associated with the regulation of astrocyte polarization and the suppression of demyelination in hydrocephalic mice, which may be a potential therapeutic strategy for NPH.
Keywords: N-acetylmannosamine; N-acetylneuraminic acid; hydrocephalus; metabolomics.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Das JM, Biagioni MC. Normal pressure hydrocephalus. StatPearls [Internet]. 2021. - PubMed
-
- Martín‐Láez R, Caballero‐Arzapalo H, López‐Menéndez L, Arango‐Lasprilla JC, Vázquez‐Barquero A. Epidemiology of idiopathic Normal pressure hydrocephalus: a systematic review of the literature. World Neurosurg. 2015;84(6):2002‐2009. - PubMed
-
- Lu VM, Kerezoudis P, Patel NP, et al. Our efforts in understanding normal pressure hydrocephalus: learning from the 100 most cited articles by bibliometric analysis. World Neurosurg. 2020;137:429‐434.e13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

