Exploring the performance of Escherichia coli outer membrane vesicles as a tool for vaccine development against Chagas disease
- PMID: 37222309
- PMCID: PMC10207902
- DOI: 10.1590/0074-02760220263
Exploring the performance of Escherichia coli outer membrane vesicles as a tool for vaccine development against Chagas disease
Abstract
Background: Vaccine development is a laborious craftwork in which at least two main components must be defined: a highly immunogenic antigen and a suitable delivery method. Hence, the interplay of these elements could elicit the required immune response to cope with the targeted pathogen with a long-lasting protective capacity.
Objectives: Here we evaluate the properties of Escherichia coli spherical proteoliposomes - known as outer membrane vesicles (OMVs) - as particles with natural adjuvant capacities and as antigen-carrier structures to assemble an innovative prophylactic vaccine for Chagas disease.
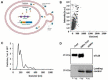
Methods: To achieve this, genetic manipulation was carried out on E. coli using an engineered plasmid containing the Tc24 Trypanosoma cruzi antigen. The goal was to induce the release of OMVs displaying the parasite protein on their surface.
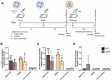
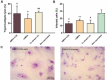
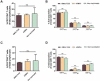
Findings: As a proof of principle, we observed that native OMVs - as well as those carrying the T. cruzi antigen - were able to trigger a slight, but functional humoral response at low immunization doses. Of note, compared to the non-immunized group, native OMVs-vaccinated animals survived the lethal challenge and showed minor parasitemia values, suggesting a possible involvement of innate trained immunity mechanism.
Main conclusion: These results open the range for further research on the design of new carrier strategies focused on innate immunity activation as an additional immunization target and venture to seek for alternative forms in which OMVs could be used for optimizing vaccine development.
Figures
Similar articles
-
Expression, purification, immunogenicity, and protective efficacy of a recombinant Tc24 antigen as a vaccine against Trypanosoma cruzi infection in mice.Vaccine. 2015 Aug 26;33(36):4505-12. doi: 10.1016/j.vaccine.2015.07.017. Epub 2015 Jul 17. Vaccine. 2015. PMID: 26192358
-
Experimental Chagas disease in Balb/c mice previously vaccinated with T. rangeli. II. The innate immune response shows immunological memory: reality or fiction?Immunobiology. 2015 Mar;220(3):428-36. doi: 10.1016/j.imbio.2014.10.003. Epub 2014 Oct 30. Immunobiology. 2015. PMID: 25454810
-
Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection.Sci Rep. 2016 Nov 16;6:37242. doi: 10.1038/srep37242. Sci Rep. 2016. PMID: 27849050 Free PMC article.
-
The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease.Expert Rev Mol Med. 2010 Sep 15;12:e29. doi: 10.1017/S1462399410001560. Expert Rev Mol Med. 2010. PMID: 20840799 Review.
-
Chagas disease vaccine design: the search for an efficient Trypanosoma cruzi immune-mediated control.Biochim Biophys Acta Mol Basis Dis. 2020 May 1;1866(5):165658. doi: 10.1016/j.bbadis.2019.165658. Epub 2020 Jan 3. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31904415 Review.
Cited by
-
Protective Efficacy of the Epitope-Conjugated Antigen N-Tc52/TSkb20 in Mitigating Trypanosoma cruzi Infection through CD8+ T-Cells and IFNγ Responses.Vaccines (Basel). 2024 Jun 4;12(6):621. doi: 10.3390/vaccines12060621. Vaccines (Basel). 2024. PMID: 38932350 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

