Cyclic-di-AMP signalling in lactic acid bacteria
- PMID: 37222477
- PMCID: PMC10243994
- DOI: 10.1093/femsre/fuad025
Cyclic-di-AMP signalling in lactic acid bacteria
Abstract
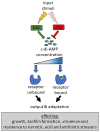
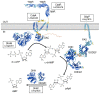
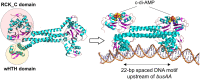
Cyclic dimeric adenosine monophosphate (cyclic-di-AMP) is a nucleotide second messenger present in Gram-positive bacteria, Gram-negative bacteria and some Archaea. The intracellular concentration of cyclic-di-AMP is adjusted in response to environmental and cellular cues, primarily through the activities of synthesis and degradation enzymes. It performs its role by binding to protein and riboswitch receptors, many of which contribute to osmoregulation. Imbalances in cyclic-di-AMP can lead to pleiotropic phenotypes, affecting aspects such as growth, biofilm formation, virulence, and resistance to osmotic, acid, and antibiotic stressors. This review focuses on cyclic-di-AMP signalling in lactic acid bacteria (LAB) incorporating recent experimental discoveries and presenting a genomic analysis of signalling components from a variety of LAB, including those found in food, and commensal, probiotic, and pathogenic species. All LAB possess enzymes for the synthesis and degradation of cyclic-di-AMP, but are highly variable with regards to the receptors they possess. Studies in Lactococcus and Streptococcus have revealed a conserved function for cyclic-di-AMP in inhibiting the transport of potassium and glycine betaine, either through direct binding to transporters or to a transcriptional regulator. Structural analysis of several cyclic-di-AMP receptors from LAB has also provided insights into how this nucleotide exerts its influence.
Keywords: compatible solutes; cyclic-di-AMP; lactic acid bacteria; osmotic stress; potassium.
© The Author(s) 2023. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures


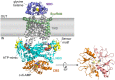

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

