Simulation of Membrane Fabrication via Solvent Evaporation and Nonsolvent-Induced Phase Separation
- PMID: 37222486
- PMCID: PMC10739593
- DOI: 10.1021/acsami.3c03126
Simulation of Membrane Fabrication via Solvent Evaporation and Nonsolvent-Induced Phase Separation
Erratum in
-
Correction to "Simulation of Membrane Fabrication via Solvent Evaporation and Nonsolvent-Induced Phase Separation".ACS Appl Mater Interfaces. 2024 Mar 6;16(9):12115. doi: 10.1021/acsami.4c02290. Epub 2024 Feb 21. ACS Appl Mater Interfaces. 2024. PMID: 38381780 Free PMC article. No abstract available.
Abstract
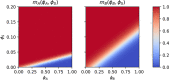
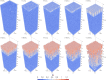
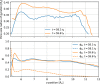
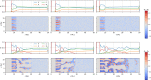
Block copolymer membranes offer a bottom-up approach to form isoporous membranes that are useful for ultrafiltration of functional macromolecules, colloids, and water purification. The fabrication of isoporous block copolymer membranes from a mixed film of an asymmetric block copolymer and two solvents involves two stages: First, the volatile solvent evaporates, creating a polymer skin, in which the block copolymer self-assembles into a top layer, comprised of perpendicularly oriented cylinders, via evaporation-induced self-assembly (EISA). This top layer imparts selectivity onto the membrane. Subsequently, the film is brought into contact with a nonsolvent, and the exchange between the remaining nonvolatile solvent and nonsolvent through the self-assembled top layer results in nonsolvent-induced phase separation (NIPS). Thereby, a macroporous support for the functional top layer that imparts mechanical stability onto the system without significantly affecting permeability is fabricated. We use a single, particle-based simulation technique to investigate the sequence of both processes, EISA and NIPS. The simulations identify a process window, which allows for the successful in silico fabrication of integral-asymmetric, isoporous diblock copolymer membranes, and provide direct insights into the spatiotemporal structure formation and arrest. The role of the different thermodynamic (e.g., solvent selectivity for the block copolymer components) and kinetic (e.g., plasticizing effect of the solvent) characteristics is discussed.
Keywords: copolymer membranes; evaporation-induced self-assembly; micro- and macrophase separation; nonsolvent-induced phase separation; simulation and modeling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










Similar articles
-
Toward Predicting the Formation of Integral-Asymmetric, Isoporous Diblock Copolymer Membranes.Adv Mater. 2024 Oct;36(40):e2404560. doi: 10.1002/adma.202404560. Epub 2024 Aug 29. Adv Mater. 2024. PMID: 39206611
-
Nonequilibrium Processes in Polymer Membrane Formation: Theory and Experiment.Chem Rev. 2021 Nov 24;121(22):14189-14231. doi: 10.1021/acs.chemrev.1c00029. Epub 2021 May 25. Chem Rev. 2021. PMID: 34032399
-
Influence of Solvent on the Structure of an Amphiphilic Block Copolymer in Solution and in Formation of an Integral Asymmetric Membrane.ACS Appl Mater Interfaces. 2017 Sep 20;9(37):31224-31234. doi: 10.1021/acsami.6b15199. Epub 2017 Feb 15. ACS Appl Mater Interfaces. 2017. PMID: 28199082
-
Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development.Membranes (Basel). 2021 Apr 23;11(5):309. doi: 10.3390/membranes11050309. Membranes (Basel). 2021. PMID: 33922560 Free PMC article. Review.
-
Phase-Field Simulation of the Effect of Coagulation Bath Temperature on the Structure and Properties of Polyvinylidene Fluoride Microporous Membranes Prepared by a Nonsolvent-Induced Phase Separation.ACS Omega. 2022 Dec 29;8(1):180-189. doi: 10.1021/acsomega.2c06983. eCollection 2023 Jan 10. ACS Omega. 2022. PMID: 36643498 Free PMC article. Review.
Cited by
-
Nonsolvent-Induced Phase Separation-Jet Spinning: An Innovative Technique for Producing Cellulosic Nanofilms, Suspensions, and Nanofilm-Based Sponges.ACS Omega. 2025 Aug 1;10(31):34389-34398. doi: 10.1021/acsomega.5c02353. eCollection 2025 Aug 12. ACS Omega. 2025. PMID: 40821602 Free PMC article.
-
GPU Accelerated Hybrid Particle-Field Molecular Dynamics: Multi-Node/Multi-GPU Implementation and Large-Scale Benchmarks of the OCCAM Code.J Comput Chem. 2025 May 15;46(13):e70126. doi: 10.1002/jcc.70126. J Comput Chem. 2025. PMID: 40365831 Free PMC article.
References
-
- Drioli E.; Brunetti A.; Di Profio G.; Barbieri G. Process Intensification Strategies and Membrane Engineering. Green Chem. 2012, 14, 1561–1572. 10.1039/c2gc16668b. - DOI
-
- Sanders D. F.; Smith Z. P.; Guo R.; Robeson L. M.; McGrath J. E.; Paul D. R.; Freeman B. D. Energy-Efficient Polymeric Gas Separation Membranes for a Sustainable Future: A Review. Polymer 2013, 54, 4729–4761. 10.1016/j.polymer.2013.05.075. - DOI
Publication types
LinkOut - more resources
Full Text Sources

