Comparison of indirect treatment methods in migraine prevention to address differences in mode of administration
- PMID: 37222593
- PMCID: PMC10508308
- DOI: 10.57264/cer-2023-0021
Comparison of indirect treatment methods in migraine prevention to address differences in mode of administration
Abstract
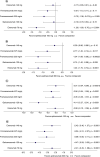
Aim: Indirect treatment comparisons (ITCs) are anchored on a placebo comparator, and the placebo response may vary according to drug administration route. Migraine preventive treatment studies were used to evaluate ITCs and determine whether mode of administration influences placebo response and the overall study findings. Materials & methods: Change from baseline in monthly migraine days produced by monoclonal antibody treatments (subcutaneous, intravenous) was compared using fixed-effects Bayesian network meta-analysis (NMA), network meta-regression (NMR), and unanchored simulated treatment comparison (STC). Results: NMA and NMR provide mixed, rarely differentiated results between treatments, whereas unanchored STC strongly favors eptinezumab over other preventive treatments. Conclusion: Further investigations are needed to determine which ITC best reflects the impact of mode of administration on placebo.
Keywords: indirect treatment comparisons; intravenous; migraine; mode of administration; network meta-analysis; network meta-regression; placebo response; subcutaneous; unanchored simulated treatment comparison.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


Similar articles
-
Preventive drug treatments for adults with chronic migraine: a systematic review with economic modelling.Health Technol Assess. 2024 Oct;28(63):1-329. doi: 10.3310/AYWA5297. Health Technol Assess. 2024. PMID: 39365169 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
Cited by
-
Comparative effectiveness of erenumab versus rimegepant for migraine prevention using matching-adjusted indirect comparison.J Comp Eff Res. 2024 Mar;13(3):e230122. doi: 10.57264/cer-2023-0122. Epub 2024 Jan 4. J Comp Eff Res. 2024. PMID: 38174577 Free PMC article.
-
Network connectivity, between-study heterogeneity and timepoint challenges in generalized myasthenia gravis: a feasibility assessment of indirect treatment comparisons.J Comp Eff Res. 2025 Jun;14(6):e250009. doi: 10.57264/cer-2025-0009. Epub 2025 May 5. J Comp Eff Res. 2025. PMID: 40321129 Free PMC article.
-
Are indirect comparisons for treatments in migraine necessitas? Many inevitable challenges to overcome.J Comp Eff Res. 2023 Jul;12(7):e230082. doi: 10.57264/cer-2023-0082. Epub 2023 Jun 2. J Comp Eff Res. 2023. PMID: 37265067 Free PMC article. No abstract available.
References
-
- O'Rourke B, Oortwijn W, Schuller T. The New Definition of Health Technology Assessment: a Milestone in International Collaboration. Cambridge University Press, Cambridge, UK: (2020). - PubMed
-
- Es-Skali IJ, Spoors J. Analysis of indirect treatment comparisons in national health technology assessments and requirements for industry submissions. J. Comp. Eff. Res. 7(4), 397–409 (2018). - PubMed
-
- Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Bayesian methods in health technology assessment: a review. Health Technol. Assess (Rockv) 4(38), 1–130 (2000). - PubMed
-
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. National Institute for Health and Care Excellence ; Royston, UK: (2011). - PubMed
-
- Vase L, Vollert J, Finnerup NB et al. Predictors of the placebo analgesia response in randomized controlled trials of chronic pain: a meta-analysis of the individual data from nine industrially sponsored trials. Pain 156(9), 1795–1802 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
