A C. albicans TRAPP Complex-Associated Gene Contributes to Cell Wall Integrity, Hyphal and Biofilm Formation, and Tissue Invasion
- PMID: 37222596
- PMCID: PMC10269527
- DOI: 10.1128/spectrum.05361-22
A C. albicans TRAPP Complex-Associated Gene Contributes to Cell Wall Integrity, Hyphal and Biofilm Formation, and Tissue Invasion
Abstract
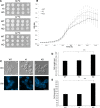
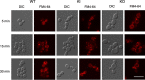
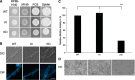
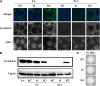
While endocytic and secretory pathways are well-studied cellular processes in the model yeast Saccharomyces cerevisiae, they remain understudied in the opportunistic fungal pathogen Candida albicans. We previously found that null mutants of C. albicans homologs of the S. cerevisiae early endocytosis genes ENT2 and END3 not only exhibited delayed endocytosis but also had defects in cell wall integrity, filamentation, biofilm formation, extracellular protease activity, and tissue invasion in an in vitro model. In this study, we focused on a potential C. albicans homolog to S. cerevisiae TCA17, which was discovered in our whole-genome bioinformatics approach aimed at identifying genes involved in endocytosis. In S. cerevisiae, TCA17 encodes a transport protein particle (TRAPP) complex-associated protein. Using a reverse genetics approach with CRISPR-Cas9-mediated gene deletion, we analyzed the function of the TCA17 homolog in C. albicans. Although the C. albicans tca17Δ/Δ null mutant did not have defects in endocytosis, it displayed an enlarged cell and vacuole morphology, impaired filamentation, and reduced biofilm formation. Moreover, the mutant exhibited altered sensitivity to cell wall stressors and antifungal agents. When assayed using an in vitro keratinocyte infection model, virulence properties were also diminished. Our findings indicate that C. albicans TCA17 may be involved in secretion-related vesicle transport and plays a role in cell wall and vacuolar integrity, hyphal and biofilm formation, and virulence. IMPORTANCE The fungal pathogen Candida albicans causes serious opportunistic infections in immunocompromised patients and has become a major cause of hospital-acquired bloodstream infections, catheter-associated infections, and invasive disease. However, due to a limited understanding of Candida molecular pathogenesis, clinical approaches for the prevention, diagnosis, and treatment of invasive candidiasis need significant improvement. In this study, we focus on identifying and characterizing a gene potentially involved in the C. albicans secretory pathway, as intracellular transport is critical for C. albicans virulence. We specifically investigated the role of this gene in filamentation, biofilm formation, and tissue invasion. Ultimately, these findings advance our current understanding of C. albicans biology and may have implications for the diagnosis and treatment of candidiasis.
Keywords: Candida albicans; TRAPP complex; biofilm; filamentation; pathogenesis; secretion; trafficking; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M, Wilson D, Hube B. 2012. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One 7:e36952. doi:10.1371/journal.pone.0036952. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

