Ketoconazole as second-line treatment for Cushing's disease after transsphenoidal surgery: systematic review and meta-analysis
- PMID: 37223017
- PMCID: PMC10200879
- DOI: 10.3389/fendo.2023.1145775
Ketoconazole as second-line treatment for Cushing's disease after transsphenoidal surgery: systematic review and meta-analysis
Abstract
Introduction: The first-line treatment for Cushing's disease is transsphenoidal surgery for pituitary tumor resection. Ketoconazole has been used as a second-line drug despite limited data on its safety and efficacy for this purpose. The objective of this meta-analysis was to analyze hypercortisolism control in patients who used ketoconazole as a second-line treatment after transsphenoidal surgery, in addition to other clinical and laboratory criteria that could be related to therapeutic response.
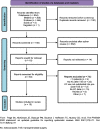
Methods: We searched for articles that evaluated ketoconazole use in Cushing's disease after transsphenoidal surgery. The search strategies were applied to MEDLINE, EMBASE, and SciELO. Independent reviewers assessed study eligibility and quality and extracted data on hypercortisolism control and related variables such as therapeutic dose, time, and urinary cortisol levels.
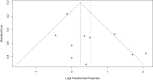
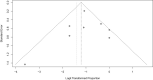
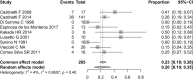
Results: After applying the exclusion criteria, 10 articles (one prospective and nine retrospective studies, totaling 270 patients) were included for complete data analysis. We found no publication bias regarding reported biochemical control or no biochemical control (p = 0.06 and p = 0.42 respectively). Of 270 patients, biochemical control of hypercortisolism occurred in 151 (63%, 95% CI 50-74%) and no biochemical control occurred in 61 (20%, 95% CI 10-35%). According to the meta-regression, neither the final dose, treatment duration, nor initial serum cortisol levels were associated with biochemical control of hypercortisolism.
Conclusion: Ketoconazole can be considered a safe and efficacious option for Cushing's disease treatment after pituitary surgery.
Systematic review registration: https://www.crd.york.ac.uk/prospero/#searchadvanced, (CRD42022308041).
Keywords: Cushing’s disease; ketoconazole; meta-analysis; systematic review; treatment.
Copyright © 2023 Viecceli, Mattos, Hirakata, Garcia, Rodrigues and Czepielewski.
Conflict of interest statement
TCR received a CNPQ research grant. MAC worked on clinical research for Crinetics and on the advisory board for Novo Nordisk. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

