New insights into signal transduction pathways in adrenal steroidogenesis: role of mitochondrial fusion, lipid mediators, and MAPK phosphatases
- PMID: 37223023
- PMCID: PMC10200866
- DOI: 10.3389/fendo.2023.1175677
New insights into signal transduction pathways in adrenal steroidogenesis: role of mitochondrial fusion, lipid mediators, and MAPK phosphatases
Abstract
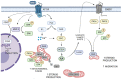
Hormone-receptor signal transduction has been extensively studied in adrenal gland. Zona glomerulosa and fasciculata cells are responsible for glucocorticoid and mineralocorticoid synthesis by adrenocorticotropin (ACTH) and angiotensin II (Ang II) stimulation, respectively. Since the rate-limiting step in steroidogenesis occurs in the mitochondria, these organelles are key players in the process. The maintenance of functional mitochondria depends on mitochondrial dynamics, which involves at least two opposite events, i.e., mitochondrial fusion and fission. This review presents state-of-the-art data on the role of mitochondrial fusion proteins, such as mitofusin 2 (Mfn2) and optic atrophy 1 (OPA1), in Ang II-stimulated steroidogenesis in adrenocortical cells. Both proteins are upregulated by Ang II, and Mfn2 is strictly necessary for adrenal steroid synthesis. The signaling cascades of steroidogenic hormones involve an increase in several lipidic metabolites such as arachidonic acid (AA). In turn, AA metabolization renders several eicosanoids released to the extracellular medium able to bind membrane receptors. This report discusses OXER1, an oxoeicosanoid receptor which has recently arisen as a novel participant in adrenocortical hormone-stimulated steroidogenesis through its activation by AA-derived 5-oxo-ETE. This work also intends to broaden knowledge of phospho/dephosphorylation relevance in adrenocortical cells, particularly MAP kinase phosphatases (MKPs) role in steroidogenesis. At least three MKPs participate in steroid production and processes such as the cellular cycle, either directly or by means of MAP kinase regulation. To sum up, this review discusses the emerging role of mitochondrial fusion proteins, OXER1 and MKPs in the regulation of steroid synthesis in adrenal cortex cells.
Keywords: MKP-3; OXER1; adrenocortical cells; mitochondrial fusion; mitofusin 2; oxoeicosanoids; protein kinases; steroidogenesis.
Copyright © 2023 Mori Sequeiros Garcia, Paz, Castillo, Benzo, Belluno, Balcázar Martínez, Maloberti, Cornejo Maciel and Poderoso.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

