Diagnostic testing for interstitial lung disease in common variable immunodeficiency: a systematic review
- PMID: 37223103
- PMCID: PMC10200864
- DOI: 10.3389/fimmu.2023.1190235
Diagnostic testing for interstitial lung disease in common variable immunodeficiency: a systematic review
Abstract
Introduction: Common variable immunodeficiency related interstitial lung disease (CVID-ILD, also referred to as GLILD) is generally considered a manifestation of systemic immune dysregulation occurring in up to 20% of people with CVID. There is a lack of evidence-based guidelines for the diagnosis and management of CVID-ILD.
Aim: To systematically review use of diagnostic tests for assessing patients with CVID for possible ILD, and to evaluate their utility and risks.
Methods: EMBASE, MEDLINE, PubMed and Cochrane databases were searched. Papers reporting information on the diagnosis of ILD in patients with CVID were included.
Results: 58 studies were included. Radiology was the investigation modality most commonly used. HRCT was the most reported test, as abnormal radiology often first raised suspicion of CVID-ILD. Lung biopsy was used in 42 (72%) of studies, and surgical lung biopsy had more conclusive results compared to trans-bronchial biopsy (TBB). Analysis of broncho-alveolar lavage was reported in 24 (41%) studies, primarily to exclude infection. Pulmonary function tests, most commonly gas transfer, were widely used. However, results varied from normal to severely impaired, typically with a restrictive pattern and reduced gas transfer.
Conclusion: Consensus diagnostic criteria are urgently required to support accurate assessment and monitoring in CVID-ILD. ESID and the ERS e-GLILDnet CRC have initiated a diagnostic and management guideline through international collaboration.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022276337.
Keywords: CVID; GLILD; diagnosis; interstitial lung disease; systematic review.
Copyright © 2023 Bintalib, van de Ven, Jacob, Davidsen, Fevang, Hanitsch, Malphettes, van Montfrans, Maglione, Milito, Routes, Warnatz and Hurst.
Conflict of interest statement
AV reports fees for educational activities from Takeda outside the submitted work.JJ reports fees from Boehringer Ingelheim, Roche, NHSX, Takeda and GlaxoSmithKline unrelated to the submitted work. JJ was supported by Wellcome Trust Clinical Research Career Development Fellowship 209553/Z/17/Z and the NIHR Biomedical Research Centre at University College London.JD reports fees for advisory board meetings, teaching and educational activities, and congress participation from Boehringer Ingelheim outside the submitted work.PM has received grant support from the National Institutes of Health, AAAAI Foundation, Immune Deficiency Foundation, Takeda, Horizon Pharma, and Boston University and has received consulting fees from Medscape and Pharming.KW reports honoraria for advisory board meetings, teaching and educational activities from TAKEDA, LFB biomedicaments, CSL Behring, Grifols, and Bristol-Myers Squibb outside the submitted work. In addition, KW has received a research grant by Bristol-Myers Squibb for the investigation of Abatacept for interstitial lung disease in CVID.JH has received support to attend meetings, personal payment and payment to his employer from companies that make medicines to treat respiratory disease and immunoglobulin products. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

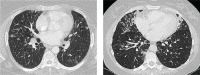
References
-
- Fraz MSA, Michelsen AE, Moe N, Aaløkken TM, Macpherson ME, Nordøy I, et al. . Raised serum markers of T cell activation and exhaustion in granulomatous-lymphocytic interstitial lung disease in common variable immunodeficiency. J Clin Immunol (2022) 42:1–11. doi: 10.1007/s10875-022-01318-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

