Exploring the Effect of Pincer Rigidity on Oxidative Addition Reactions with Cobalt(I) Complexes
- PMID: 37223209
- PMCID: PMC10201995
- DOI: 10.1021/acs.organomet.3c00079
Exploring the Effect of Pincer Rigidity on Oxidative Addition Reactions with Cobalt(I) Complexes
Abstract
Cobalt complexes containing the 2,6-diaminopyridine-substituted PNP pincer (iPrPNMeNP = 2,6-(iPr2PNMe)2(C5H3N)) were synthesized. A combination of solid-state structures and investigation of the cobalt(I)/(II) redox potential established a relatively rigid and electron-donating chelating ligand as compared to iPrPNP (iPrPNP = 2,6-(iPr2PCH2)2(C5H3N)). Based on a buried volume analysis, the two pincer ligands are sterically indistinguishable. Nearly planar, diamagnetic, four-coordinate complexes were observed independent of the field strength (chloride, alkyl, aryl) of the fourth ligand completing the coordination sphere of the metal. Computational studies supported a higher barrier for C-H oxidative addition, largely a result of the increased rigidity of the pincer. The increased oxidative addition barrier resulted in stabilization of (iPrPNMeNP)Co(I) complexes, enabling the characterization of the cobalt boryl and the cobalt hydride dimer by X-ray crystallography. Moreover, (iPrPNMeNP)CoMe served as an efficient precatalyst for alkene hydroboration likely because of the reduced propensity to undergo oxidative addition, demonstrating that reactivity and catalytic performance can be tuned by rigidity of pincer ligands.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
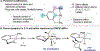









References
-
- van Koten G Tuning the Reactivity of Metals Held in a Rigid Ligand Environment. Pure Appl. Chem. 1989, 61, 1681–1694.
-
- Morales-Morales D Pincer Compounds: Chemistry and Applications, 1st ed.; Elsevier, 2018.
-
- Kelly WSJ; Ford GH; Nelson SM Studies on the Magnetic Crossover in Five-Co-ordinate Complexes of Iron(II), Cobalt(II), and Nickel(II). Part I. J. Chem. Soc. A, 1971, 388–396.
- Dahlhoff WV; Nelson SM. Studies on the Magnetic Cross-over in Five-Co-ordinate Complexes of Iron(II), Cobalt(II), and Nickel(II). Part II. J. Chem. Soc. A, 1971, 2184–2190.
-
- Moulton CJ; Shaw BL Transition Metal–Carbon Bonds. Part XLII. Complexes of Nickel, Palladium, Platinum, Rhodium and Iridium with the Tridentate Ligand 2,6-Bis[(di-t-butylphosphino)methyl]phenyl. J. Chem. Soc., Dalton Trans. 1976, 1020–1024.
-
- Dupont J; Consorti CS; Spencer J Organometallic Pincer-Type Complexes: Recent Applications in Synthesis and Catalysis. In The Chemistry of Pincer Compounds, 1st ed.; Morales-Morales D, Jensen CM. Eds.; Elsevier Science: Amsterdam, 2007; pp 1–24.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
