Neisseria gonorrhoeae-derived outer membrane vesicles package β-lactamases to promote antibiotic resistance
- PMID: 37223348
- PMCID: PMC10117772
- DOI: 10.1093/femsml/uqac013
Neisseria gonorrhoeae-derived outer membrane vesicles package β-lactamases to promote antibiotic resistance
Abstract
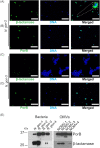
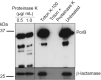
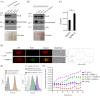
Neisseria gonorrhoeae causes the sexually transmitted disease gonorrhoea. The treatment of gonorrhoea is becoming increasingly challenging, as N. gonorrhoeae has developed resistance to antimicrobial agents routinely used in the clinic. Resistance to penicillin is wide-spread partly due to the acquisition of β-lactamase genes. How N. gonorrhoeae survives an initial exposure to β-lactams before acquiring resistance genes remains to be understood. Here, using a panel of clinical isolates of N. gonorrhoeae we show that the β-lactamase enzyme is packaged into outer membrane vesicles (OMVs) by strains expressing blaTEM-1B or blaTEM-106, which protects otherwise susceptible clinical isolates from the β-lactam drug amoxycillin. We characterized the phenotypes of these clinical isolates of N. gonorrhoeae and the time courses over which the cross-protection of the strains is effective. Imaging and biochemical assays suggest that OMVs promote the transfer of proteins and lipids between bacteria. Thus, N. gonorrhoeae strains secret antibiotic degrading enzymes via OMVs enabling survival of otherwise susceptible bacteria.
Keywords: OMV; TEM-1; antimicrobial resistance; gonorrhoea; lactamase; secretion system.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures


References
-
- Ashford WA, Golash RG, Hemming VG.. Penicillinase-producing Neisseria gonorrhoeae. Lancet North Am Ed. 1976;308:657–8. - PubMed
-
- Bala M, Kakran M, Singh Vet al. Monitoring antimicrobial resistance in Neisseria gonorrhoeae in selected countries of the WHO South-East Asia Region between 2009 and 2012: a retrospective analysis. Sex Transm Infect. 2013;89:iv28–iv35. - PubMed
LinkOut - more resources
Full Text Sources
