Eubacterium rectale Improves the Efficacy of Anti-PD1 Immunotherapy in Melanoma via l-Serine-Mediated NK Cell Activation
- PMID: 37223471
- PMCID: PMC10202379
- DOI: 10.34133/research.0127
Eubacterium rectale Improves the Efficacy of Anti-PD1 Immunotherapy in Melanoma via l-Serine-Mediated NK Cell Activation
Abstract
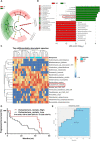
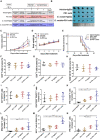
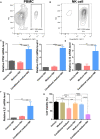
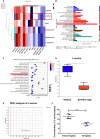
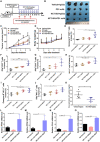
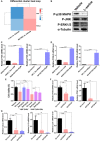
Natural killer (NK) cells, as key immune cells, play essential roles in tumor cell immune escape and immunotherapy. Accumulating evidence has demonstrated that the gut microbiota community affects the efficacy of anti-PD1 immunotherapy and that remodeling the gut microbiota is a promising strategy to enhance anti-PD1 immunotherapy responsiveness in advanced melanoma patients; however, the details of the mechanism remain elusive. In this study, we found that Eubacterium rectale was significantly enriched in melanoma patients who responded to anti-PD1 immunotherapy and that a high E. rectale abundance was related to longer survival in melanoma patients. Furthermore, administration of E. rectale remarkably improved the efficacy of anti-PD1 therapy and increased the overall survival of tumor-bearing mice; moreover, application of E. rectale led to a significant accumulation of NK cells in the tumor microenvironment. Interestingly, conditioned medium isolated from an E. rectale culture system dramatically enhanced NK cell function. Gas chromatography-mass spectrometry/ultrahigh performance liquid chromatography-tandem mass spectrometry-based metabolomic analysis showed that l-serine production was significantly decreased in the E. rectale group; moreover, administration of an l-serine synthesis inhibitor dramatically increased NK cell activation, which enhanced anti-PD1 immunotherapy effects. Mechanistically, supplementation with l-serine or application of an l-serine synthesis inhibitor affected NK cell activation through Fos/Fosl. In summary, our findings reveal the role of bacteria-modulated serine metabolic signaling in NK cell activation and provide a novel therapeutic strategy to improve the efficacy of anti-PD1 immunotherapy in melanoma.
Copyright © 2023 Nian Liu et al.
Figures

Similar articles
-
DPP inhibition alters the CXCR3 axis and enhances NK and CD8+ T cell infiltration to improve anti-PD1 efficacy in murine models of pancreatic ductal adenocarcinoma.J Immunother Cancer. 2021 Nov;9(11):e002837. doi: 10.1136/jitc-2021-002837. J Immunother Cancer. 2021. PMID: 34737215 Free PMC article.
-
Eravacycline improves the efficacy of anti-PD1 immunotherapy via AP1/CCL5 mediated M1 macrophage polarization in melanoma.Biomaterials. 2025 Mar;314:122815. doi: 10.1016/j.biomaterials.2024.122815. Epub 2024 Sep 11. Biomaterials. 2025. PMID: 39288620
-
An Engineered IL15 Cytokine Mutein Fused to an Anti-PD1 Improves Intratumoral T-cell Function and Antitumor Immunity.Cancer Immunol Res. 2021 Oct;9(10):1141-1157. doi: 10.1158/2326-6066.CIR-21-0058. Epub 2021 Aug 10. Cancer Immunol Res. 2021. PMID: 34376502
-
Gut Microbiota: A Promising Milestone in Enhancing the Efficacy of PD1/PD-L1 Blockade Therapy.Front Oncol. 2022 Feb 16;12:847350. doi: 10.3389/fonc.2022.847350. eCollection 2022. Front Oncol. 2022. PMID: 35252014 Free PMC article. Review.
-
NK cells and ILCs in tumor immunotherapy.Mol Aspects Med. 2021 Aug;80:100870. doi: 10.1016/j.mam.2020.100870. Epub 2020 Aug 13. Mol Aspects Med. 2021. PMID: 32800530 Review.
Cited by
-
Lachnospiraceae are emerging industrial biocatalysts and biotherapeutics.Front Bioeng Biotechnol. 2024 Jan 4;11:1324396. doi: 10.3389/fbioe.2023.1324396. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38239921 Free PMC article. Review.
-
Metabolic regulation of the immune system in health and diseases: mechanisms and interventions.Signal Transduct Target Ther. 2024 Oct 9;9(1):268. doi: 10.1038/s41392-024-01954-6. Signal Transduct Target Ther. 2024. PMID: 39379377 Free PMC article. Review.
-
Gut microbiome: decision-makers in the microenvironment of colorectal cancer.Front Cell Infect Microbiol. 2023 Dec 12;13:1299977. doi: 10.3389/fcimb.2023.1299977. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38156313 Free PMC article. Review.
-
The role of the microscopic world: Exploring the role and potential of intratumoral microbiota in cancer immunotherapy.Medicine (Baltimore). 2024 May 17;103(20):e38078. doi: 10.1097/MD.0000000000038078. Medicine (Baltimore). 2024. PMID: 38758914 Free PMC article. Review.
-
Anti-inflammatory Effects of Membrane Vesicles from Eubacterium rectale via the NLRP3 Signal Pathway.Probiotics Antimicrob Proteins. 2024 Dec 20. doi: 10.1007/s12602-024-10432-y. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 39702738
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. - PubMed
-
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. . Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. - PubMed
-
- Roy S, Trinchieri G. Microbiota: A key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–285. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

