SARS-CoV-2 RBD and Its Variants Can Induce Platelet Activation and Clearance: Implications for Antibody Therapy and Vaccinations against COVID-19
- PMID: 37223472
- PMCID: PMC10202384
- DOI: 10.34133/research.0124
SARS-CoV-2 RBD and Its Variants Can Induce Platelet Activation and Clearance: Implications for Antibody Therapy and Vaccinations against COVID-19
Abstract
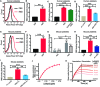
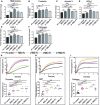
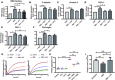
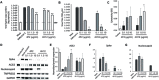
The COVID-19 pandemic caused by SARS-CoV-2 virus is an ongoing global health burden. Severe cases of COVID-19 and the rare cases of COVID-19 vaccine-induced-thrombotic-thrombocytopenia (VITT) are both associated with thrombosis and thrombocytopenia; however, the underlying mechanisms remain inadequately understood. Both infection and vaccination utilize the spike protein receptor-binding domain (RBD) of SARS-CoV-2. We found that intravenous injection of recombinant RBD caused significant platelet clearance in mice. Further investigation revealed the RBD could bind platelets, cause platelet activation, and potentiate platelet aggregation, which was exacerbated in the Delta and Kappa variants. The RBD-platelet interaction was partially dependent on the β3 integrin as binding was significantly reduced in β3-/- mice. Furthermore, RBD binding to human and mouse platelets was significantly reduced with related αIIbβ3 antagonists and mutation of the RGD (arginine-glycine-aspartate) integrin binding motif to RGE (arginine-glycine-glutamate). We developed anti-RBD polyclonal and several monoclonal antibodies (mAbs) and identified 4F2 and 4H12 for their potent dual inhibition of RBD-induced platelet activation, aggregation, and clearance in vivo, and SARS-CoV-2 infection and replication in Vero E6 cells. Our data show that the RBD can bind platelets partially though αIIbβ3 and induce platelet activation and clearance, which may contribute to thrombosis and thrombocytopenia observed in COVID-19 and VITT. Our newly developed mAbs 4F2 and 4H12 have potential not only for diagnosis of SARS-CoV-2 virus antigen but also importantly for therapy against COVID-19.
Copyright © 2023 Xiaoying Ma et al.
Figures


References
-
- Ciceri F, Beretta L, Scandroglio AM, Colombo S, Landoni G, Ruggeri A, Peccatori J, D’Angelo A, De Cobelli F, Rovere-Querini P, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(2):95–97. - PMC - PubMed
-
- Boeckh-Behrens T, Golkowski D, Ikenberg B, Schlegel J, Protzer U, Schulz C, Novotny J, Kreiser K, Zimmer C, Hemmer B, et al. COVID-19-associated large vessel stroke in a 28-year-old patient : NETs and platelets possible key players in acute thrombus formation. Clin Neuroradiol. 2021;31(2):511–514. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

