Recent advances and perspectives in nucleotide second messenger signaling in bacteria
- PMID: 37223732
- PMCID: PMC10118264
- DOI: 10.1093/femsml/uqad015
Recent advances and perspectives in nucleotide second messenger signaling in bacteria
Abstract
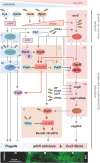
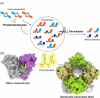
Nucleotide second messengers act as intracellular 'secondary' signals that represent environmental or cellular cues, i.e. the 'primary' signals. As such, they are linking sensory input with regulatory output in all living cells. The amazing physiological versatility, the mechanistic diversity of second messenger synthesis, degradation, and action as well as the high level of integration of second messenger pathways and networks in prokaryotes has only recently become apparent. In these networks, specific second messengers play conserved general roles. Thus, (p)ppGpp coordinates growth and survival in response to nutrient availability and various stresses, while c-di-GMP is the nucleotide signaling molecule to orchestrate bacterial adhesion and multicellularity. c-di-AMP links osmotic balance and metabolism and that it does so even in Archaea may suggest a very early evolutionary origin of second messenger signaling. Many of the enzymes that make or break second messengers show complex sensory domain architectures, which allow multisignal integration. The multiplicity of c-di-GMP-related enzymes in many species has led to the discovery that bacterial cells are even able to use the same freely diffusible second messenger in local signaling pathways that can act in parallel without cross-talking. On the other hand, signaling pathways operating with different nucleotides can intersect in elaborate signaling networks. Apart from the small number of common signaling nucleotides that bacteria use for controlling their cellular "business," diverse nucleotides were recently found to play very specific roles in phage defense. Furthermore, these systems represent the phylogenetic ancestors of cyclic nucleotide-activated immune signaling in eukaryotes.
Keywords: Ap4A; CBASS; biofilm; c-di-AMP; c-di-GMP; cGAMP; ppGpp.
© The Author(s) 2023. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
