Development of relugolix combination therapy as a medical treatment option for women with uterine fibroids or endometriosis
- PMID: 37223761
- PMCID: PMC10201285
- DOI: 10.1016/j.xfre.2022.11.010
Development of relugolix combination therapy as a medical treatment option for women with uterine fibroids or endometriosis
Abstract
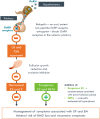
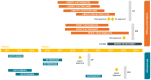
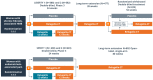
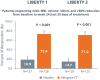
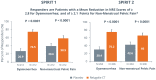
Treatment of uterine fibroids (UF) and endometriosis (EM) has relied on the surgical skills of gynecologists to improve symptoms and potentially alter the course of these debilitating diseases. Medical management of symptoms for both diseases leverages combined hormonal contraceptives used off label as a first-line treatment, with nonsteroid anti-inflammatory drugs and opioids to manage pain as needed. Gonadotropin-releasing hormone (GnRH) receptor agonists (peptide analogs) have been used as short-term therapy to manage severe symptoms of UF or EM, treat anemia, and reduce fibroid size before surgery. The introduction of oral GnRH receptor antagonists opened the door for the development of new treatment options for UF, EM, and other estrogen-driven diseases. Relugolix is an orally active, nonpeptide, GnRH receptor antagonist that competitively binds to GnRH receptors, preventing the release of follicle-stimulating hormone and luteinizing hormone (LH) into the systemic circulation. In women, the reduction in follicle-stimulating hormone concentrations prevents natural follicular development, suppressing ovarian production of estrogen, and together with reductions in LH concentrations, prevent ovulation, corpus luteum formation, and, thereby, the production of progesterone (P). By reducing circulating concentrations of estradiol (E2) and P, relugolix improves heavy menstrual bleeding and other symptoms associated with UF and moderate to severe pain associated with EM, including dysmenorrhea, nonmenstrual pelvic pain (NMPP) and dyspareunia. However, as monotherapy, the use of relugolix is associated with signs and symptoms of a hypoestrogenic state, including bone mineral density loss and vasomotor symptoms. The clinical development of relugolix incorporated the addition of a 1 mg dose of E2 and a 0.5-mg dose of norethindrone acetate (NETA) to achieve systemic E2 concentrations that remain in a therapeutic range while mitigating the risk for bone mineral density loss and vasomotor symptoms, enabling the longer-term treatment and reducing the impact of symptoms on quality of life, and potentially delaying or preventing the need for surgery. Relugolix 40 mg in combination with estradiol (E2) 1 mg and NETA 0.5 mg as a single fixed-dose combination tablet (relugolix combination therapy [relugolix-CT]) approved in the United States as MYFEMBREE is the first and only once daily oral GnRH antagonist combination therapy indicated for the management of heavy menstrual bleeding associated with UF and moderate to severe pain associated with EM. In the European Union (EU) and the United Kingdom (UK), relugolix-CT is approved as RYEQO for the management of symptoms associated with UF. In Japan, relugolix 40 mg, as monotherapy, was the first GnRH receptor antagonist approved to improve symptoms associated with UF or pain associated with EM under the brand name RELUMINA. In men, relugolix suppresses testosterone production. Relugolix 120 mg (ORGOVYX) was developed by Myovant Sciences and is approved in the United States, EU, and UK as the first and only oral androgen-deprivation therapy for the treatment of advanced prostate cancer. This review is focused on the development of relugolix and relugolix-CT in women's health indications.
Keywords: GnRH antagonist; add-back therapy; bone mineral density loss; endometriosis; relugolix; uterine fibroids.
© 2022 The Authors.
Figures


Similar articles
-
Relugolix/Estradiol/Norethisterone Acetate: A Review in Endometriosis-Associated Pain.Drugs. 2024 Apr;84(4):449-457. doi: 10.1007/s40265-024-02018-3. Epub 2024 Apr 9. Drugs. 2024. PMID: 38592603 Free PMC article. Review.
-
Relugolix/Estradiol/Norethisterone (Norethindrone) Acetate: A Review in Symptomatic Uterine Fibroids.Drugs. 2022 Oct;82(15):1549-1556. doi: 10.1007/s40265-022-01790-4. Epub 2022 Nov 4. Drugs. 2022. PMID: 36331779 Free PMC article. Review.
-
Two-year efficacy and safety of relugolix combination therapy in women with endometriosis-associated pain: SPIRIT open-label extension study.Hum Reprod. 2024 Mar 1;39(3):526-537. doi: 10.1093/humrep/dead263. Hum Reprod. 2024. PMID: 38243752 Free PMC article. Clinical Trial.
-
An evaluation of relugolix/estradiol/norethindrone acetate for the treatment of heavy menstrual bleeding associated with uterine fibroids in premenopausal women.Expert Opin Pharmacother. 2022 Mar;23(4):421-429. doi: 10.1080/14656566.2022.2030705. Epub 2022 Jan 24. Expert Opin Pharmacother. 2022. PMID: 35068291 Free PMC article.
-
Treatment of Uterine Fibroid Symptoms with Relugolix Combination Therapy.N Engl J Med. 2021 Feb 18;384(7):630-642. doi: 10.1056/NEJMoa2008283. N Engl J Med. 2021. PMID: 33596357 Free PMC article. Clinical Trial.
Cited by
-
Novel Approaches to Possible Targeted Therapies and Prophylaxis of Uterine Fibroids.Diseases. 2023 Nov 1;11(4):156. doi: 10.3390/diseases11040156. Diseases. 2023. PMID: 37987267 Free PMC article. Review.
-
Safety and Tolerability of Relugolix in Combination with Abiraterone or Apalutamide for Treatment of Patients with Advanced Prostate Cancer: Data from a 52-Week Clinical Trial.Target Oncol. 2025 May;20(3):503-517. doi: 10.1007/s11523-025-01139-3. Epub 2025 Apr 4. Target Oncol. 2025. PMID: 40180682 Free PMC article. Clinical Trial.
-
A Podcast on Patient and Physician Perspectives on the Management of Endometriosis and Relugolix Combination Therapy.Adv Ther. 2024 Dec;41(12):4369-4376. doi: 10.1007/s12325-024-02970-2. Epub 2024 Oct 19. Adv Ther. 2024. PMID: 39425891 Free PMC article.
-
Relugolix/Estradiol/Norethisterone Acetate: A Review in Endometriosis-Associated Pain.Drugs. 2024 Apr;84(4):449-457. doi: 10.1007/s40265-024-02018-3. Epub 2024 Apr 9. Drugs. 2024. PMID: 38592603 Free PMC article. Review.
-
Management of Abnormal Uterine Bleeding Among Reproductive Age Group Women: A Cross-Sectional Study.J Clin Med. 2024 Nov 23;13(23):7086. doi: 10.3390/jcm13237086. J Clin Med. 2024. PMID: 39685546 Free PMC article.
References
-
- Zondervan K.T., Becker C.M., Missmer S.A. Endometriosis. N Engl J Med. 2020;382:1244–1256. - PubMed
-
- Nelson A.L., Ritchie J.J. Severe anemia from heavy menstrual bleeding requires heightened attention. Am J Obstet Gynecol. 2015;213 97.e1–97.e6. - PubMed
-
- Dmowski W.P., Lesniewicz R., Rana N., Pepping P., Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67:238–243. - PubMed
LinkOut - more resources
Full Text Sources

