Ganirelix and the prevention of premature luteinizing hormone surges
- PMID: 37223764
- PMCID: PMC10201301
- DOI: 10.1016/j.xfre.2023.02.009
Ganirelix and the prevention of premature luteinizing hormone surges
Abstract
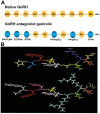
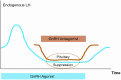
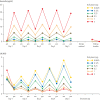
Ganirelix is a gonadotropin-releasing hormone (GnRH) antagonist with high antagonistic activity that blocks the GnRH receptor by competitive binding. A daily dose of 0.25 mg of ganirelix was sel5ected after a phase II study because it was the minimal, effective daily dose to prevent premature luteinizing hormone surges and this dose yielded the highest ongoing pregnancy rate per started cycle. After subcutaneous administration, ganirelix is rapidly absorbed, reaching peak levels within 1-2 hours (tmax), and has a high absolute bioavailability (>90%). Prospective, comparative studies have demonstrated the advantages of GnRH antagonists over long GnRH agonist treatment in assisted reproduction, including the immediate reversibility of drug effects, a requirement for less follicle-stimulating hormone, a shortened duration of stimulation, a reduced incidence of ovarian hyperstimulation syndrome, and reduced patient burden. Combined analyses concluded that in the general in vitro fertilization population, there is a trend for a slightly lower ongoing pregnancy rate and a lower risk of ovarian hyperstimulation syndrome that is largely eliminated when considering triggering with GnRH agonist instead of human chorionic gonadotropin. Regardless of all the research, it is still not fully elucidated why the long GnRH agonist protocol has a trend for higher pregnancy rates after fresh transfer of the same number of good-quality embryos.
Keywords: GnRH antagonist; OHSS; ganirelix; pregnancy rates; prevention of premature LH surges.
© 2023 The Author(s).
Figures
Similar articles
-
Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndrome: preliminary report: short communication.Hum Reprod. 2000 Sep;15(9):1965-8. doi: 10.1093/humrep/15.9.1965. Hum Reprod. 2000. PMID: 10966996
-
Comparable clinical outcome using the GnRH antagonist ganirelix or a long protocol of the GnRH agonist triptorelin for the prevention of premature LH surges in women undergoing ovarian stimulation.Hum Reprod. 2001 Apr;16(4):644-51. doi: 10.1093/humrep/16.4.644. Hum Reprod. 2001. PMID: 11278211 Clinical Trial.
-
A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon). The ganirelix dose-finding study group.Hum Reprod. 1998 Nov;13(11):3023-31. Hum Reprod. 1998. PMID: 9853849 Clinical Trial.
-
The prevention of ovarian hyperstimulation syndrome.J Obstet Gynaecol Can. 2014 Nov;36(11):1024-1033. doi: 10.1016/S1701-2163(15)30417-5. J Obstet Gynaecol Can. 2014. PMID: 25574681 Review. English, French.
-
Ovarian stimulation for in-vitro fertilization/intracytoplasmic sperm injection with gonadotrophins and gonadotrophin-releasing hormone analogues: agonists and antagonists.Hum Reprod. 1999 Sep;14 Suppl 1:207-21. doi: 10.1093/humrep/14.suppl_1.207. Hum Reprod. 1999. PMID: 10573035 Review.
Cited by
-
GnRH Peptide Antagonist: Comparative Analysis of Chemistry and Formulation with Implications for Clinical Safety and Efficacy.Pharmaceuticals (Basel). 2024 Dec 31;18(1):36. doi: 10.3390/ph18010036. Pharmaceuticals (Basel). 2024. PMID: 39861098 Free PMC article. Review.
-
The role of elagolix in ovulation suppression during controlled ovarian stimulation: a retrospective cohort study.F S Rep. 2024 Jul 4;5(4):356-362. doi: 10.1016/j.xfre.2024.06.006. eCollection 2024 Dec. F S Rep. 2024. PMID: 39781082 Free PMC article.
References
-
- Mannaerts B., Gordon K. Embryo implantation and GnRH antagonists: GnRH antagonists do not activate the GnRH receptor. Hum Reprod. 2000;15:1882–1883. - PubMed
-
- A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon). The ganirelix dose-finding study group. Hum Reprod. 1998;13:3023–3031. - PubMed
-
- Kol S., Lightman A., Hillensjö T., Devroey P., Fauser B., Tarlatzis B., et al. High doses of gonadotrophin-releasing hormone antagonist in in-vitro fertilization cycles do not adversely affect the outcome of subsequent freeze-thaw cycles. Hum Reprod. 1999;14:2242–2244. - PubMed
-
- Simon C., Oberyé J., Bellver J., Vidal C., Bosch E., Horcajadas J.A., et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod. 2005;20:3318–3327. - PubMed
-
- Oberyé J.J.L., Mannaerts B.M.J.L., Kleijn H.J., Timmer C.J. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part I. Absolute bioavailability of 0.25 mg of ganirelix after a single subcutaneous injection in healthy female volunteers. Fertil Steril. 1999;72:1001–1005. - PubMed
LinkOut - more resources
Full Text Sources

