Clinical development of the oral gonadotropin-releasing hormone antagonist elagolix
- PMID: 37223767
- PMCID: PMC10201304
- DOI: 10.1016/j.xfre.2023.02.002
Clinical development of the oral gonadotropin-releasing hormone antagonist elagolix
Abstract
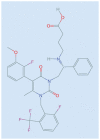
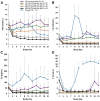
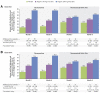
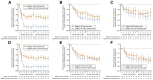
Elagolix is the first oral gonadotropin-releasing hormone antagonist that entered clinical development and received regulatory approval for the management of women with endometriosis and heavy menstrual bleeding associated with uterine fibroids in combination with a hormonal add-back therapy. This mini review aims to summarize the key clinical studies that led to its regulatory approval.
Keywords: Elagolix; GnRH antagonist; endometriosis; uterine fibroids.
© 2023 The Author.
Figures
References
-
- Ng J., Chwalisz K., Carter D.C., Klein C.E. Dose-dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J Clin Endocrinol Metab. 2017;102:1683–1691. - PubMed
-
- Millar R.P., Zhu Y.F., Chen C., Struthers R.S. Progress towards the development of non-peptide orally-active gonadotropin-releasing hormone (GnRH) antagonists: therapeutic implications. Br Med Bull. 2000;56:761–772. - PubMed
-
- Tucci F.C., Zhu Y.F., Struthers R.S., Guo Z., Gross T.D., Rowbottom M.W., et al. 3-[(2R)-amino-2-phenylethyl]-1-(2,6-difluorobenzyl)-5-(2-fluoro-3-methoxyphenyl)- 6-methylpyrimidin-2,4-dione (NBI 42902) as a potent and orally active antagonist of the human gonadotropin-releasing hormone receptor. Design, synthesis, and in vitro and in vivo characterization. J Med Chem. 2005;48:1169–1178. - PubMed
-
- Chen C., Wu D., Guo Z., Xie Q., Reinhart G.J., Madan A., et al. Discovery of sodium R-(+)-4-{2-[5-(2-fluoro-3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino}butyrate (elagolix), a potent and orally available nonpeptide antagonist of the human gonadotropin-releasing hormone receptor. J Med Chem. 2008;51:7478–7485. - PubMed
-
- Taylor H.S., Dun E.C., Chwalisz K. Clinical evaluation of the oral gonadotropin-releasing hormone-antagonist elagolix for the management of endometriosis-associated pain. Pain Manag. 2019;9:497–515. - PubMed
LinkOut - more resources
Full Text Sources

