Simulation of drug-drug interactions between breast cancer chemotherapeutic agents and antiemetic drugs
- PMID: 37223851
- PMCID: PMC10624783
- DOI: 10.1007/s40199-023-00463-1
Simulation of drug-drug interactions between breast cancer chemotherapeutic agents and antiemetic drugs
Abstract
Background: Chemotherapy-induced nausea and vomiting are commonly experienced side effects in breast cancer (BCa) patients. Antiemetic drugs used in BCa treatment are either inhibitors or inducers of cytochrome P450 (CYP) enzymes, while anticancer drugs are metabolized by CYPs.
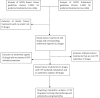
Objectives: The purpose of the present work was to evaluate in silico drug-drug interaction (DDI) potential between BCa chemotherapeutic drugs and antiemetic agents.
Methods: The Drug-Drug Interaction™ module of GastroPlus™ was employed to assess CYP-related interactions between antiemetic and anticancer therapy combinations. The CYP inhibitory or inducing parameters (IC50, Ki, EC50) used in simulations were obtained from the literature.
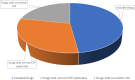
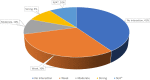
Results: Analyses of twenty-three BCa drugs indicated that 22% of the chemotherapeutic drugs do not need an antiemetic agent due to their low emetogenicity, whereas 30% of the anticancer drugs are not metabolized by CYPs. The remaining eleven anticancer drugs metabolized by CYPs generated ninety-nine combinations with nine antiemetics. Simulation of DDIs suggest that about half of the pairs did not demonstrate any potential for DDI, whereas 30%, 10%, and 9% of the pairs showed weak, moderate, and strong interaction potential, respectively. In the present study, netupitant was the only antiemetic that showed strong inhibitory interactions (predicted AUC ratio > 5) with CYP3A4-metabolzied anticancer therapies (e.g., docetaxel, ribociclib, olaparib). Moderate to no interactions were observed with ondansetron, aprepitant, rolapitant, and dexamethasone in combination with anticancer agents.
Conclusion: It is critical to recognize that these interactions can get amplified in cancer patients because of the severity of the disease and chemotherapy toxicities. Clinicians need to be aware of the DDI likelihood of the drug combinations used in BCa treatment.
Keywords: Antiemetics; Chemotherapeutic agents; Cytochrome P450; Drug-drug interaction; Simulation.
© 2023. The Author(s), under exclusive licence to Tehran University of Medical Sciences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Opioid-induced respiratory depression suspected of drug interaction in a prostate cancer patient: a case report.Ann Palliat Med. 2024 Mar;13(2):428-432. doi: 10.21037/apm-23-581. Ann Palliat Med. 2024. PMID: 38584476
-
Drug-drug interactions with aprepitant in antiemetic prophylaxis for chemotherapy.Neth J Med. 2018 Apr;76(3):109-114. Neth J Med. 2018. PMID: 29667586 Review.
-
Aprepitant when added to a standard antiemetic regimen consisting of ondansetron and dexamethasone does not affect vinorelbine pharmacokinetics in cancer patients.Cancer Chemother Pharmacol. 2007 Feb;59(3):407-12. doi: 10.1007/s00280-006-0359-6. Epub 2006 Oct 19. Cancer Chemother Pharmacol. 2007. PMID: 17051369
-
Clinical pharmacology of neurokinin-1 receptor antagonists for the treatment of nausea and vomiting associated with chemotherapy.Expert Opin Drug Saf. 2017 Jun;16(6):697-710. doi: 10.1080/14740338.2017.1325868. Expert Opin Drug Saf. 2017. PMID: 28460548 Review.
-
A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy.Breast Cancer Res Treat. 2009 Feb;113(3):529-35. doi: 10.1007/s10549-008-9957-9. Epub 2008 Mar 10. Breast Cancer Res Treat. 2009. PMID: 18327706 Clinical Trial.
References
-
- Adel N. Overview of chemotherapy-induced nausea and vomiting and evidence-based therapies. Am J Manag Care. 2017;23(14 Suppl):S259–SS65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

