Therapeutic Potential of HMF and Its Derivatives: a Computational Study
- PMID: 37223872
- PMCID: PMC10206368
- DOI: 10.1007/s12010-023-04547-1
Therapeutic Potential of HMF and Its Derivatives: a Computational Study
Abstract
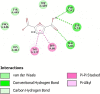
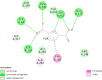
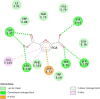
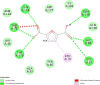
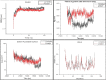
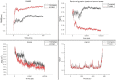
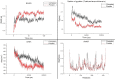
Over the past century, chemicals and energy have increasingly been derived from non-renewable resources. The growing demand for essential chemicals and shrinking inventory make reliable, sustainable sources essential. Carbohydrates offer by far the greatest carbon supply. Furan compounds, a particular family of dehydration products, are believed to offer high chemical potential. Here, we analyze 5-HMF (5, hydroxymethylfurfural) and some of its derivatives in particular, a furan-type platform chemical. To analyze the therapeutic potential of HMF and its derivatives, this study utilized cutting-edge technologies such as computer-aided drug design, virtual screening, molecular docking, and molecular dynamic simulation. We conducted 189 docking simulations and examined some of the most promising dock poses using the molecular dynamic simulator. As for the receptors for our compounds, the leading candidates are human acetylcholinesterase, beta-lactamases, P. aeruginosa LasR, and S. aureus tyrosyl-tRNA synthetases. Out of all derivatives considered in this study, 2,5-furandicarboxylic acid (FCA) performed best.
Keywords: Antimicrobial; Docking; HMF; MD Simulation; Therapeutic.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
One-Pot Conversion of Carbohydrates into Furan Derivatives via Furfural and 5-Hydroxylmethylfurfural as Intermediates.ChemSusChem. 2016 Aug 23;9(16):2015-36. doi: 10.1002/cssc.201600507. Epub 2016 Jul 11. ChemSusChem. 2016. PMID: 27396713 Review.
-
Diverse Applications of Biomass-Derived 5-Hydroxymethylfurfural and Derivatives as Renewable Starting Materials.ChemSusChem. 2022 Jul 7;15(13):e202200328. doi: 10.1002/cssc.202200328. Epub 2022 Jun 2. ChemSusChem. 2022. PMID: 35652539 Review.
-
Advances in enzymatic conversion of biomass derived furfural and 5-hydroxymethylfurfural to value-added chemicals and solvents.Bioresour Technol. 2023 Jun;378:128975. doi: 10.1016/j.biortech.2023.128975. Epub 2023 Mar 27. Bioresour Technol. 2023. PMID: 36990330 Review.
-
Bioconversion of 5-Hydroxymethylfurfural (HMF) to 2,5-Furandicarboxylic Acid (FDCA) by a Native Obligate Aerobic Bacterium, Acinetobacter calcoaceticus NL14.Appl Biochem Biotechnol. 2020 Oct;192(2):455-465. doi: 10.1007/s12010-020-03325-7. Epub 2020 May 11. Appl Biochem Biotechnol. 2020. PMID: 32394319
-
Purification of biomass-derived 5-hydroxymethylfurfural and its catalytic conversion to 2,5-furandicarboxylic Acid.ChemSusChem. 2014 Aug;7(8):2131-5. doi: 10.1002/cssc.201402105. Epub 2014 May 28. ChemSusChem. 2014. PMID: 24889713
Cited by
-
In Silico Bioprospection of Daniellia oliveri-Based Products as Quorum Sensing Modulators of Escherichia coli SdiA.Biochem Res Int. 2025 Jun 9;2025:7191508. doi: 10.1155/bri/7191508. eCollection 2025. Biochem Res Int. 2025. PMID: 40524946 Free PMC article.
References
-
- Aresta M, Dibenedetto A (2019) Beyond fractionation in the utilization of microalgal components. Bioenergy with carbon capture and storage: Using natural resources for sustainable development 173–193 10.1016/B978-0-12-816229-3.00009-0
-
- Morone A, Apte M, Pandey RA. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renewable and Sustainable Energy Reviews. 2015;51:548–565. doi: 10.1016/j.rser.2015.06.032. - DOI
-
- Neves P, Lima S, Pillinger M, et al. Conversion of furfuryl alcohol to ethyl levulinate using porous aluminosilicate acid catalysts. Catalysis Today. 2013;218–219:76–84. doi: 10.1016/J.CATTOD.2013.04.035. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

