Non-muscle-invasive bladder cancer molecular subtypes predict differential response to intravesical Bacillus Calmette-Guérin
- PMID: 37224225
- PMCID: PMC10572776
- DOI: 10.1126/scitranslmed.abn4118
Non-muscle-invasive bladder cancer molecular subtypes predict differential response to intravesical Bacillus Calmette-Guérin
Abstract
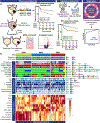
The recommended treatment for patients with high-risk non-muscle-invasive bladder cancer (HR-NMIBC) is tumor resection followed by adjuvant Bacillus Calmette-Guérin (BCG) bladder instillations. However, only 50% of patients benefit from this therapy. If progression to advanced disease occurs, then patients must undergo a radical cystectomy with risks of substantial morbidity and poor clinical outcome. Identifying tumors unlikely to respond to BCG can translate into alternative treatments, such as early radical cystectomy, targeted therapies, or immunotherapies. Here, we conducted molecular profiling of 132 patients with BCG-naive HR-NMIBC and 44 patients with recurrences after BCG (34 matched), which uncovered three distinct BCG response subtypes (BRS1, 2 and BRS3). Patients with BRS3 tumors had a reduced recurrence-free and progression-free survival compared with BRS1/2. BRS3 tumors expressed high epithelial-to-mesenchymal transition and basal markers and had an immunosuppressive profile, which was confirmed with spatial proteomics. Tumors that recurred after BCG were enriched for BRS3. BRS stratification was validated in a second cohort of 151 BCG-naive patients with HR-NMIBC, and the molecular subtypes outperformed guideline-recommended risk stratification based on clinicopathological variables. For clinical application, we confirmed that a commercially approved assay was able to predict BRS3 tumors with an area under the curve of 0.87. These BCG response subtypes will allow for improved identification of patients with HR-NMIBC at the highest risk of progression and have the potential to be used to select more appropriate treatments for patients unlikely to respond to BCG.
Conflict of interest statement
Figures




Comment in
-
A Step Closer to Predicting Progression After Bacillus Calmette-Guérin Immunotherapy in High-risk Non-muscle-invasive Bladder Cancer.Eur Urol. 2023 Nov;84(5):447-448. doi: 10.1016/j.eururo.2023.06.017. Epub 2023 Jul 1. Eur Urol. 2023. PMID: 37400353
References
-
- Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Seisen T, Soukup V, Sylvester RJ, European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol, (2021); published online EpubSep 9 ( 10.1016/j.eururo.2021.08.010). - DOI - PubMed
-
- Oddens J, Brausi M, Sylvester R, Bono A, van de Beek C, van Andel G, Gontero P, Hoeltl W, Turkeri L, Marreaud S, Collette S, Oosterlinck W, Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 63, 462–472 (2013); published online EpubMar ( 10.1016/j.eururo.2012.10.039). - DOI - PubMed
-
- de Jong FC, Hoedemaeker RF, Kvikstad V, Mensink JTM, de Jong JJ, Boevé ER, van der Schoot DKE, Zwarthoff EC, Boormans JL, Zuiverloon TCM, T1 Substaging of Nonmuscle Invasive Bladder Cancer is Associated with bacillus Calmette-Guérin Failure and Improves Patient Stratification at Diagnosis. J Urol, 101097ju0000000000001422 (2020); published online EpubNov 16 ( 10.1097/ju.0000000000001422). - DOI - PubMed
-
- Pietzak EJ, Zabor EC, Bagrodia A, Armenia J, Hu W, Zehir A, Funt S, Audenet F, Barron D, Maamouri N, Li Q, Teo MY, Arcila ME, Berger MF, Schultz N, Dalbagni G, Herr HW, Bajorin DF, Rosenberg JE, Al-Ahmadie H, Bochner BH, Solit DB, Iyer G, Genomic Differences Between “Primary” and “Secondary” Muscle-invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-based Neoadjuvant Chemotherapy. Eur Urol 75, 231–239 (2019); published online EpubFeb ( - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

