Multiomic signals associated with maternal epidemiological factors contributing to preterm birth in low- and middle-income countries
- PMID: 37224249
- PMCID: PMC10208584
- DOI: 10.1126/sciadv.ade7692
Multiomic signals associated with maternal epidemiological factors contributing to preterm birth in low- and middle-income countries
Abstract
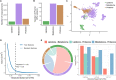
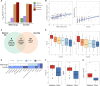
Preterm birth (PTB) is the leading cause of death in children under five, yet comprehensive studies are hindered by its multiple complex etiologies. Epidemiological associations between PTB and maternal characteristics have been previously described. This work used multiomic profiling and multivariate modeling to investigate the biological signatures of these characteristics. Maternal covariates were collected during pregnancy from 13,841 pregnant women across five sites. Plasma samples from 231 participants were analyzed to generate proteomic, metabolomic, and lipidomic datasets. Machine learning models showed robust performance for the prediction of PTB (AUROC = 0.70), time-to-delivery (r = 0.65), maternal age (r = 0.59), gravidity (r = 0.56), and BMI (r = 0.81). Time-to-delivery biological correlates included fetal-associated proteins (e.g., ALPP, AFP, and PGF) and immune proteins (e.g., PD-L1, CCL28, and LIFR). Maternal age negatively correlated with collagen COL9A1, gravidity with endothelial NOS and inflammatory chemokine CXCL13, and BMI with leptin and structural protein FABP4. These results provide an integrated view of epidemiological factors associated with PTB and identify biological signatures of clinical covariates affecting this disease.
Figures




References
-
- Chawanpaiboon S., Vogel J. P., Moller A.-B., Lumbiganon P., Petzold M., Hogan D., Landoulsi S., Jampathong N., Kongwattanakul K., Laopaiboon M., Lewis C., Rattanakanokchai S., Teng D. N., Thinkhamrop J., Watananirun K., Zhang J., Zhou W., Gülmezoglu A. M., Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 7, e37–e46 (2019). - PMC - PubMed
-
- Saigal S., Doyle L. W., An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008). - PubMed
-
- Vogel J. P., Chawanpaiboon S., Moller A.-B., Watananirun K., Bonet M., Lumbiganon P., The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 52, 3–12 (2018). - PubMed
-
- Peterson L. S., Stelzer I. A., Tsai A. S., Ghaemi M. S., Han X., Ando K., Winn V. D., Martinez N. R., Contrepois K., Moufarrej M. N., Quake S., Relman D. A., Snyder M. P., Shaw G. M., Stevenson D. K., Wong R. J., Arck P., Angst M. S., Aghaeepour N., Gaudilliere B., Multiomic immune clockworks of pregnancy. Semin. Immunopathol. 42, 397–412 (2020). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

